Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection
- PMID: 22348029
- PMCID: PMC3278405
- DOI: 10.1371/journal.pone.0030904
Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection
Abstract
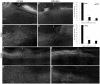
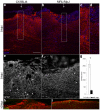
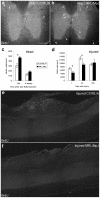
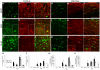
Adult MRL/MpJ mice have been shown to possess unique regeneration capabilities. They are able to heal an ear-punched hole or an injured heart with normal tissue architecture and without scar formation. Here we present functional and histological evidence for enhanced recovery following spinal cord injury (SCI) in MRL/MpJ mice. A control group (C57BL/6 mice) and MRL/MpJ mice underwent a dorsal hemisection at T9 (thoracic vertebra 9). Our data show that MRL/MpJ mice recovered motor function significantly faster and more completely. We observed enhanced regeneration of the corticospinal tract (CST). Furthermore, we observed a reduced astrocytic response and fewer micro-cavities at the injury site, which appear to create a more growth-permissive environment for the injured axons. Our data suggest that the reduced astrocytic response is in part due to a lower lesion-induced increase of cell proliferation post-SCI, and a reduced astrocytic differentiation of the proliferating cells. Interestingly, we also found an increased number of proliferating microglia, which could be involved in the MRL/MpJ spinal cord repair mechanisms. Finally, to evaluate the molecular basis of faster spinal cord repair, we examined the difference in gene expression changes in MRL/MpJ and C57BL/6 mice after SCI. Our microarray data support our histological findings and reveal a transcriptional profile associated with a more efficient spinal cord repair in MRL/MpJ mice.
Conflict of interest statement
Figures
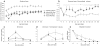
References
-
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. - PubMed
-
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. - PubMed
-
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. - PubMed
-
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

