Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress
- PMID: 22348036
- PMCID: PMC3278440
- DOI: 10.1371/journal.pone.0031017
Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress
Abstract
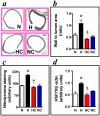
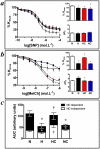
Fetal hypoxia is a common complication of pregnancy. It has been shown to programme cardiac and endothelial dysfunction in the offspring in adult life. However, the mechanisms via which this occurs remain elusive, precluding the identification of potential therapy. Using an integrative approach at the isolated organ, cellular and molecular levels, we tested the hypothesis that oxidative stress in the fetal heart and vasculature underlies the molecular basis via which prenatal hypoxia programmes cardiovascular dysfunction in later life. In a longitudinal study, the effects of maternal treatment of hypoxic (13% O(2)) pregnancy with an antioxidant on the cardiovascular system of the offspring at the end of gestation and at adulthood were studied. On day 6 of pregnancy, rats (n = 20 per group) were exposed to normoxia or hypoxia ± vitamin C. At gestational day 20, tissues were collected from 1 male fetus per litter per group (n = 10). The remaining 10 litters per group were allowed to deliver. At 4 months, tissues from 1 male adult offspring per litter per group were either perfusion fixed, frozen, or dissected for isolated organ preparations. In the fetus, hypoxic pregnancy promoted aortic thickening with enhanced nitrotyrosine staining and an increase in cardiac HSP70 expression. By adulthood, offspring of hypoxic pregnancy had markedly impaired NO-dependent relaxation in femoral resistance arteries, and increased myocardial contractility with sympathetic dominance. Maternal vitamin C prevented these effects in fetal and adult offspring of hypoxic pregnancy. The data offer insight to mechanism and thereby possible targets for intervention against developmental origins of cardiac and peripheral vascular dysfunction in offspring of risky pregnancy.
Conflict of interest statement
Figures

References
-
- Basson M. Cardiovascular Disease. Nature. 2008;451(7181):903. - PubMed
-
- Agarwal A, Williams GH, Fisher NDL. Genetics of human hypertension. Trends Endocrin Metab (Review) 2005;16(3):127–133. - PubMed
-
- Barker DJP. Edinburgh: Churchill Livingstone; 1998. Mothers, Babies, and Disease in Later Life.
-
- Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

