Proteome adaptation to high temperatures in the ectothermic hydrothermal vent Pompeii worm
- PMID: 22348046
- PMCID: PMC3277501
- DOI: 10.1371/journal.pone.0031150
Proteome adaptation to high temperatures in the ectothermic hydrothermal vent Pompeii worm
Abstract
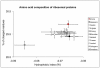
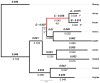
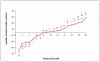
Taking advantage of the massive genome sequencing effort made on thermophilic prokaryotes, thermal adaptation has been extensively studied by analysing amino acid replacements and codon usage in these unicellular organisms. In most cases, adaptation to thermophily is associated with greater residue hydrophobicity and more charged residues. Both of these characteristics are positively correlated with the optimal growth temperature of prokaryotes. In contrast, little information has been collected on the molecular 'adaptive' strategy of thermophilic eukaryotes. The Pompeii worm A. pompejana, whose transcriptome has recently been sequenced, is currently considered as the most thermotolerant eukaryote on Earth, withstanding the greatest thermal and chemical ranges known. We investigated the amino-acid composition bias of ribosomal proteins in the Pompeii worm when compared to other lophotrochozoans and checked for putative adaptive changes during the course of evolution using codon-based Maximum likelihood analyses. We then provided a comparative analysis of codon usage and amino-acid replacements from a greater set of orthologous genes between the Pompeii worm and Paralvinella grasslei, one of its closest relatives living in a much cooler habitat. Analyses reveal that both species display the same high GC-biased codon usage and amino-acid patterns favoring both positively-charged residues and protein hydrophobicity. These patterns may be indicative of an ancestral adaptation to the deep sea and/or thermophily. In addition, the Pompeii worm displays a set of amino-acid change patterns that may explain its greater thermotolerance, with a significant increase in Tyr, Lys and Ala against Val, Met and Gly. Present results indicate that, together with a high content in charged residues, greater proportion of smaller aliphatic residues, and especially alanine, may be a different path for metazoans to face relatively 'high' temperatures and thus a novelty in thermophilic metazoans.
Conflict of interest statement
Figures





References
-
- Galtier N, Lobry JR. Relationships between genomic G+C content, RNA secondary structures and optimal growth temperature in prokaryotes. J Mol Biol. 1997;44:632–636. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

