Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation
- PMID: 22348081
- PMCID: PMC3279359
- DOI: 10.1371/journal.pone.0031384
Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation
Abstract
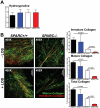
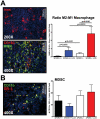
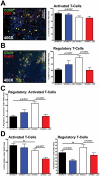
Pancreatic adenocarcinoma, a desmoplastic disease, is the fourth leading cause of cancer-related death in the Western world due, in large part, to locally invasive primary tumor growth and ensuing metastasis. SPARC is a matricellular protein that governs extracellular matrix (ECM) deposition and maturation during tissue remodeling, particularly, during wound healing and tumorigenesis. In the present study, we sought to determine the mechanism by which lack of host SPARC alters the tumor microenvironment and enhances invasion and metastasis of an orthotopic model of pancreatic cancer. We identified that levels of active TGFβ1 were increased significantly in tumors grown in SPARC-null mice. TGFβ1 contributes to many aspects of tumor development including metastasis, endothelial cell permeability, inflammation and fibrosis, all of which are altered in the absence of stromal-derived SPARC. Given these results, we performed a survival study to assess the contribution of increased TGFβ1 activity to tumor progression in SPARC-null mice using losartan, an angiotensin II type 1 receptor antagonist that diminishes TGFβ1 expression and activation in vivo. Tumors grown in SPARC-null mice progressed more quickly than those grown in wild-type littermates leading to a significant reduction in median survival. However, median survival of SPARC-null animals treated with losartan was extended to that of losartan-treated wild-type controls. In addition, losartan abrogated TGFβ induced gene expression, reduced local invasion and metastasis, decreased vascular permeability and altered the immune profile of tumors grown in SPARC-null mice. These data support the concept that aberrant TGFβ1-activation in the absence of host SPARC contributes significantly to tumor progression and suggests that SPARC, by controlling ECM deposition and maturation, can regulate TGFβ availability and activation.
Conflict of interest statement
Figures

Similar articles
-
Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC.Exp Biol Med (Maywood). 2008 Jul;233(7):860-73. doi: 10.3181/0801-RM-12. Epub 2008 Apr 29. Exp Biol Med (Maywood). 2008. PMID: 18445772 Free PMC article.
-
Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma.Dis Model Mech. 2010 Jan-Feb;3(1-2):57-72. doi: 10.1242/dmm.003228. Epub 2009 Dec 9. Dis Model Mech. 2010. PMID: 20007485 Free PMC article.
-
Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis.Mol Cancer Res. 2004 Apr;2(4):215-24. Mol Cancer Res. 2004. PMID: 15140943
-
The Influence of the Extracellular Matrix in Inflammation: Findings from the SPARC-Null Mouse.Anat Rec (Hoboken). 2020 Jun;303(6):1624-1629. doi: 10.1002/ar.24133. Epub 2019 Apr 24. Anat Rec (Hoboken). 2020. PMID: 30980479 Free PMC article. Review.
-
SPARC, a matricellular protein: at the crossroads of cell-matrix.Matrix Biol. 2000 Dec;19(7):569-80. doi: 10.1016/s0945-053x(00)00105-0. Matrix Biol. 2000. Corrected and republished in: Matrix Biol. 2001 Jan;19(8):816-27. doi: 10.1016/s0945-053x(00)00133-5. PMID: 11102747 Corrected and republished. Review.
Cited by
-
Exploring the potential role of palladin in modulating human CAF/ECM functional units.Cytoskeleton (Hoboken). 2025 Mar;82(3):175-185. doi: 10.1002/cm.21926. Epub 2024 Sep 6. Cytoskeleton (Hoboken). 2025. PMID: 39239855 Review.
-
Loss of SPARC in bladder cancer enhances carcinogenesis and progression.J Clin Invest. 2013 Feb;123(2):751-66. doi: 10.1172/JCI64782. Epub 2013 Jan 16. J Clin Invest. 2013. PMID: 23321672 Free PMC article.
-
The SPARC protein: an overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease.Br J Pharmacol. 2017 Jan;174(1):3-14. doi: 10.1111/bph.13653. Epub 2016 Nov 25. Br J Pharmacol. 2017. PMID: 27759879 Free PMC article. Review.
-
Stroma-targeting strategies in pancreatic cancer: Past lessons, challenges and prospects.World J Gastroenterol. 2021 May 14;27(18):2105-2121. doi: 10.3748/wjg.v27.i18.2105. World J Gastroenterol. 2021. PMID: 34025067 Free PMC article. Review.
-
Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels.Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. Nat Commun. 2013. PMID: 24084631 Free PMC article.
References
-
- Bornstein P. Cell-matrix interactions: the view from the outside. Methods Cell Biol. 2002;69:7–11. - PubMed
-
- Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, et al. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. - PubMed
-
- Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, et al. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:691–705. - PubMed
-
- Pen A, Moreno MJ, Martin J, Stanimirovic DB. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia. 2007;55:559–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

