Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome
- PMID: 22348120
- PMCID: PMC3279375
- DOI: 10.1371/journal.pone.0031694
Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome
Abstract
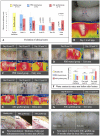
Cutaneous radiation syndrome (CRS) is the delayed consequence of localized skin exposure to high doses of ionizing radiation. Here we examined for the first time in a large animal model the therapeutic potential of autologous adipose tissue-derived stroma cells (ASCs). For experiments, Göttingen minipigs were locally gamma irradiated using a (60)Co source at the dose of 50 Gy and grafted (n = 5) or not (n = 8). ASCs were cultured in MEM-alpha with 10% fetal calf serum and basic fibroblast growth factor (2 ng.mL(-1)) and post irradiation were intradermally injected on days 25, 46, 67 and finally between days 95 and 115 (50 × 10(6) ASCs each time) into the exposed area. All controls exhibited a clinical evolution with final necrosis (day 91). In grafted pigs an ultimate wound healing was observed in four out of five grafted animals (day 130 +/- 28). Immunohistological analysis of cytokeratin expression showed a complete epidermis recovery. Grafted ASCs accumulated at the dermis/subcutis barrier in which they attracted numerous immune cells, and even an increased vasculature in one pig. Globally this study suggests that local injection of ASCs may represent a useful strategy to mitigate CRS.
Conflict of interest statement
Figures


Similar articles
-
Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig model.Exp Hematol. 2010 Oct;38(10):945-56. doi: 10.1016/j.exphem.2010.06.008. Epub 2010 Jun 25. Exp Hematol. 2010. PMID: 20600578
-
Transient gene therapy to treat cutaneous radiation syndrome: development in a minipig model.Health Phys. 2014 Jun;106(6):713-9. doi: 10.1097/HP.0000000000000099. Health Phys. 2014. PMID: 24776904
-
Contribution of INTRAMUSCULAR Autologous Adipose Tissue-Derived Stem Cell Injections to Treat Cutaneous Radiation Syndrome: Preliminary Results.Health Phys. 2016 Aug;111(2):117-26. doi: 10.1097/HP.0000000000000515. Health Phys. 2016. PMID: 27356055
-
Adipose stem cells and skin repair.Curr Stem Cell Res Ther. 2010 Jun;5(2):137-40. doi: 10.2174/157488810791268690. Curr Stem Cell Res Ther. 2010. PMID: 19941454 Review.
-
Adipose-derived stem cells for wound repair and regeneration.Expert Opin Biol Ther. 2015;15(9):1285-92. doi: 10.1517/14712598.2015.1053867. Epub 2015 Jun 3. Expert Opin Biol Ther. 2015. PMID: 26037027 Review.
Cited by
-
Persistent DNA damage after high dose in vivo gamma exposure of minipig skin.PLoS One. 2012;7(6):e39521. doi: 10.1371/journal.pone.0039521. Epub 2012 Jun 27. PLoS One. 2012. PMID: 22761813 Free PMC article.
-
Mesenchymal stem cell-based therapy for burn wound healing.Burns Trauma. 2021 May 1;9:tkab002. doi: 10.1093/burnst/tkab002. eCollection 2021. Burns Trauma. 2021. PMID: 34212055 Free PMC article. Review.
-
Cell therapy for severe burn wound healing.Burns Trauma. 2018 May 28;6:13. doi: 10.1186/s41038-018-0117-0. eCollection 2018. Burns Trauma. 2018. PMID: 29854856 Free PMC article. Review.
-
Delivery of Allogeneic Adipose Stem Cells in Polyethylene Glycol-Fibrin Hydrogels as an Adjunct to Meshed Autografts After Sharp Debridement of Deep Partial Thickness Burns.Stem Cells Transl Med. 2018 Apr;7(4):360-372. doi: 10.1002/sctm.17-0160. Epub 2018 Feb 18. Stem Cells Transl Med. 2018. PMID: 29457376 Free PMC article.
-
A new rabbit model of impaired wound healing in an X-ray-irradiated field.PLoS One. 2017 Sep 8;12(9):e0184534. doi: 10.1371/journal.pone.0184534. eCollection 2017. PLoS One. 2017. PMID: 28886194 Free PMC article.
References
-
- Lefaix JL, Delanian S. Le syndrome cutané radio-induit. In: De Revel T, Gourmelon P, Vidal D, et al., editors. Nuclear, Radiological, Biological, Chemical - Terrorist threat and medical approach, 1st ed. Montrouge: John Libbey Eurotext; 2005. pp. 87–95.
-
- Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990;57:751–773. - PubMed
-
- François S, Mouiseddine M, Mathieu N, Semont A, Monti P, et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol. 2007;86:1–8. - PubMed
-
- Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50–58. - PubMed
-
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

