SHARPIN is essential for cytokine production, NF-κB signaling, and induction of Th1 differentiation by dendritic cells
- PMID: 22348129
- PMCID: PMC3279418
- DOI: 10.1371/journal.pone.0031809
SHARPIN is essential for cytokine production, NF-κB signaling, and induction of Th1 differentiation by dendritic cells
Abstract
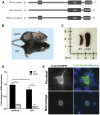
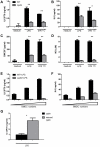
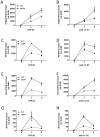
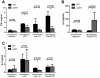
Spontaneous mutations of the Sharpin (SHANK-associated RH domain-interacting protein, other aliases: Rbckl1, Sipl1) gene in mice result in systemic inflammation that is characterized by chronic proliferative dermatitis and dysregulated secretion of T helper1 (Th1) and Th2 cytokines. The cellular and molecular mechanisms underlying this inflammatory phenotype remain elusive. Dendritic cells may contribute to the initiation and progression of the phenotype of SHARPIN-deficient mice because of their pivotal role in innate and adaptive immunity. Here we show by flow cytometry that SHARPIN- deficiency did not alter the distribution of different DC subtypes in the spleen. In response to TOLL-like receptor (TLR) agonists LPS and poly I:C, cultured bone marrow-derived dendritic cells (BMDC) from WT and mutant mice exhibited similar increases in expression of co-stimulatory molecules CD40, CD80, and CD86. However, stimulated SHARPIN-deficient BMDC had reduced transcription and secretion of pro-inflammatory mediators IL6, IL12P70, GMCSF, and nitric oxide. Mutant BMDC had defective activation of NF-κB signaling, whereas the MAPK1/3 (ERK1/2) and MAPK11/12/13/14 (p38 MAP kinase isoforms) and TBK1 signaling pathways were intact. A mixed lymphocyte reaction showed that mutant BMDC only induced a weak Th1 immune response but stimulated increased Th2 cytokine production from allogeneic naïve CD4(+) T cells. In conclusion, loss of Sharpin in mice significantly affects the immune function of DC and this may partially account for the systemic inflammation and Th2-biased immune response.
Conflict of interest statement
Figures



References
-
- Lim S, Sala C, Yoon J, Park S, Kuroda S, et al. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. Mol Cell Neurosci. 2001;17:385–397. - PubMed
-
- Seymour RE, Hasham MG, Cox GA, Shultz LD, Hogenesch H, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007;8:416–421. - PubMed
-
- HogenEsch H, Janke S, Boggess D, Sundberg JP. Absence of Peyer's patches and abnormal lymphoid architecture in chronic proliferative dermatitis (cpdm/cpdm) mice. J Immunol. 1999;162:3890–3896. - PubMed
-
- HogenEsch H, Torregrosa SE, Boggess D, Sundberg BA, Carroll J, et al. Increased expression of type 2 cytokines in chronic proliferative dermatitis (cpdm) mutant mice and resolution of inflammation following treatment with IL-12. Eur J Immunol. 2001;31:734–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

