C-terminal fluorescent labeling impairs functionality of DNA mismatch repair proteins
- PMID: 22348133
- PMCID: PMC3279419
- DOI: 10.1371/journal.pone.0031863
C-terminal fluorescent labeling impairs functionality of DNA mismatch repair proteins
Abstract
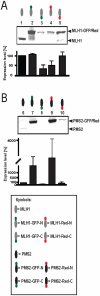
The human DNA mismatch repair (MMR) process is crucial to maintain the integrity of the genome and requires many different proteins which interact perfectly and coordinated. Germline mutations in MMR genes are responsible for the development of the hereditary form of colorectal cancer called Lynch syndrome. Various mutations mainly in two MMR proteins, MLH1 and MSH2, have been identified so far, whereas 55% are detected within MLH1, the essential component of the heterodimer MutLα (MLH1 and PMS2). Most of those MLH1 variants are pathogenic but the relevance of missense mutations often remains unclear. Many different recombinant systems are applied to filter out disease-associated proteins whereby fluorescent tagged proteins are frequently used. However, dye labeling might have deleterious effects on MutLα's functionality. Therefore, we analyzed the consequences of N- and C-terminal fluorescent labeling on expression level, cellular localization and MMR activity of MutLα. Besides significant influence of GFP- or Red-fusion on protein expression we detected incorrect shuttling of single expressed C-terminal GFP-tagged PMS2 into the nucleus and found that C-terminal dye labeling impaired MMR function of MutLα. In contrast, N-terminal tagged MutLαs retained correct functionality and can be recommended both for the analysis of cellular localization and MMR efficiency.
Conflict of interest statement
Figures




Similar articles
-
Screening for germline mutations of MLH1, MSH2, MSH6 and PMS2 genes in Slovenian colorectal cancer patients: implications for a population specific detection strategy of Lynch syndrome.Fam Cancer. 2009;8(4):421-9. doi: 10.1007/s10689-009-9258-4. Epub 2009 Jun 13. Fam Cancer. 2009. PMID: 19526325
-
Germline mutation analysis of MLH1 and MSH2 in Malaysian Lynch syndrome patients.World J Gastroenterol. 2012 Feb 28;18(8):814-20. doi: 10.3748/wjg.v18.i8.814. World J Gastroenterol. 2012. PMID: 22371642 Free PMC article.
-
Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer.Eur Urol. 2013 Feb;63(2):379-85. doi: 10.1016/j.eururo.2012.07.047. Epub 2012 Aug 2. Eur Urol. 2013. PMID: 22883484
-
Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.Eur J Cancer Prev. 2014 Jul;23(4):246-57. doi: 10.1097/CEJ.0000000000000019. Eur J Cancer Prev. 2014. PMID: 24614649 Review.
-
A review of mismatch repair gene transcripts: issues for interpretation of mRNA splicing assays.Clin Genet. 2015 Feb;87(2):100-8. doi: 10.1111/cge.12450. Epub 2014 Jul 26. Clin Genet. 2015. PMID: 24989436 Review.
Cited by
-
C-terminal eYFP fusion impairs Escherichia coli MinE function.Open Biol. 2020 May;10(5):200010. doi: 10.1098/rsob.200010. Epub 2020 May 27. Open Biol. 2020. PMID: 32456552 Free PMC article.
-
Classification of MSH6 Variants of Uncertain Significance Using Functional Assays.Int J Mol Sci. 2021 Aug 11;22(16):8627. doi: 10.3390/ijms22168627. Int J Mol Sci. 2021. PMID: 34445333 Free PMC article. Review.
-
Mismatch Repair: From Preserving Genome Stability to Enabling Mutation Studies in Real-Time Single Cells.Cells. 2021 Jun 18;10(6):1535. doi: 10.3390/cells10061535. Cells. 2021. PMID: 34207040 Free PMC article. Review.
-
An intact Pms2 ATPase domain is not essential for male fertility.DNA Repair (Amst). 2016 Mar;39:46-51. doi: 10.1016/j.dnarep.2015.12.011. Epub 2015 Dec 29. DNA Repair (Amst). 2016. PMID: 26753533 Free PMC article.
-
Determining the functional significance of mismatch repair gene missense variants using biochemical and cellular assays.Hered Cancer Clin Pract. 2012 Jul 23;10(1):9. doi: 10.1186/1897-4287-10-9. Hered Cancer Clin Pract. 2012. PMID: 22824075 Free PMC article.
References
-
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. - PubMed
-
- Mohd AB, Palama B, Nelson SE, Tomer G, Nguyen M, et al. Truncation of the C-terminus of human MLH1 blocks intracellular stabilization of PMS2 and disrupts DNA mismatch repair. DNA Repair (Amst) 2006;5:347–361. - PubMed
-
- Brieger A, Adryan B, Wolpert F, Passmann S, Zeuzem S, et al. Cytoskeletal scaffolding proteins interact with Lynch-Syndrome associated mismatch repair protein MLH1. Proteomics. 2010;10:3343–3355. - PubMed
-
- Cannavo E, Gerrits B, Marra G, Schlapbach R, Jiricny J. Characterization of the interactome of the human MutL homologues MLH1, PMS1, and PMS2. J Biol Chem. 2007;282:2976–2986. - PubMed
-
- Mac Partlin M, Homer E, Robinson H, McCormick CJ, Crouch DH, et al. Interactions of the DNA mismatch repair proteins MLH1 and MSH2 with c-MYC and MAX. Oncogene. 2003;22:819–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

