Molecular insights on pathogenic effects of mutations causing phosphoglycerate kinase deficiency
- PMID: 22348148
- PMCID: PMC3279470
- DOI: 10.1371/journal.pone.0032065
Molecular insights on pathogenic effects of mutations causing phosphoglycerate kinase deficiency
Abstract
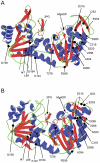
Phosphoglycerate kinase (PGK) catalyzes an important ATP-generating step in glycolysis. PGK1 deficiency is an uncommon X-linked inherited disorder, generally characterized by various combinations of non-spherocytic hemolytic anemia, neurological dysfunctions, and myopathies. Patients rarely exhibit all three clinical features. To provide a molecular framework to the different pathological manifestations, all known mutations were reviewed and 16 mutant enzymes, obtained as recombinant forms, were functionally and structurally characterized. Most mutations heavily affect thermal stability and to a different extent catalytic efficiency, in line with the remarkably low PGK activity clinically observed in the patients. Mutations grossly impairing protein stability, but moderately affecting kinetic properties (p.I47N, p.L89P, p.C316R, p.S320N, and p.A354P) present the most homogeneous correlation with the clinical phenotype. Patients carrying these mutations display hemolytic anemia and neurological disorders, and,except for p.A354P variant, no myopaty. Variants highly perturbed in both catalytic efficiency (p.G158V, p.D164V, p.K191del, D285V, p.D315N, and p.T378P) and heat stability (all, but p.T378P) result to be mainly associated with myopathy alone. Finally, mutations faintly affecting molecular properties (p.R206P, p.E252A, p.I253T, p.V266M, and p.D268N) correlate with a wide spectrum of clinical symptoms. These are the first studies that correlate the clinical symptoms with the molecular properties of the mutant enzymes. All findings indicate that the different clinical manifestations associated with PGK1 deficiency chiefly depend on the distinctive type of perturbations caused by mutations in the PGK1 gene, highlighting the need for determination of the molecular properties of PGK variants to assist in prognosis and genetic counseling. However, the clinical symptoms can not be understood only on the bases of molecular properties of the mutant enzyme. Different (environmental, metabolic, genetic and/or epigenetic) intervening factors can contribute toward the expression of PGK deficient clinical phenotypes.
Conflict of interest statement
Figures
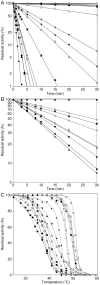
 , p.K191del; ○, p.R206P; □, p.E252A; ▵, p.I253T; ▿, p.V266M; ⋄, p.D268N;
, p.K191del; ○, p.R206P; □, p.E252A; ▵, p.I253T; ▿, p.V266M; ⋄, p.D268N;  , p.D285V;
, p.D285V;  , p.D315N;
, p.D315N;  , p.C316R;
, p.C316R;  , p.S320N;
, p.S320N;  , p.A354P;
, p.A354P;  , p.T378P.
, p.T378P.
 , p.K191del; ○, p.R206P; □, p.E252A; ▵, p.I253T; ▿, p.V266M; ⋄, p.D268N;
, p.K191del; ○, p.R206P; □, p.E252A; ▵, p.I253T; ▿, p.V266M; ⋄, p.D268N;  , p.D285V;
, p.D285V;  , p.D315N;
, p.D315N;  , p.C316R;
, p.C316R;  , p.S320N;
, p.S320N;  , p.A354P;
, p.A354P;  , p.T378P.
, p.T378P.References
-
- Beutler E. PGK deficiency. Br J Haematol. 2007;136:3–11. - PubMed
-
- Krishnan P, Gullen EA, Lam W, Dutschman GE, Grill SP, et al. Novel role of 3-phosphoglycerate kinase, a glycolytic enzyme, in the activation of L-nucleoside analogs, a new class of anticancer and antiviral agents. J Biol Chem. 2003;278:36726–36732. - PubMed
-
- Gallois-Montbrun S, Faraj A, Seclaman E, Sommadossi JP, Deville-Bonne D, et al. Broad specificity of human phosphoglycerate kinase for antiviral nucleoside analogs. Biochem Pharmacol. 2004;68:1749–1756. - PubMed
-
- Jindal HK, Vishwanatha JK. Functional identity of a primer recognition protein as phosphoglycerate kinase. J Biol Chem. 1990;265:6540–6543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

