Morphological and functional changes in the retina after chronic oxygen-induced retinopathy
- PMID: 22348151
- PMCID: PMC3279421
- DOI: 10.1371/journal.pone.0032167
Morphological and functional changes in the retina after chronic oxygen-induced retinopathy
Abstract
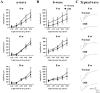
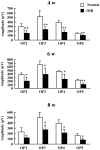
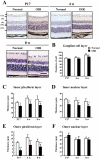
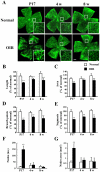
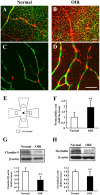
The mouse model of oxygen-induced retinopathy (OIR) has been widely used for studies of retinopathy of prematurity (ROP). This disorder, characterized by abnormal vascularization of the retina, tends to occur in low birth weight neonates after exposure to high supplemental oxygen. Currently, the incidence of ROP is increasing because of increased survival of these infants due to medical progress. However, little is known about changes in the chronic phase after ROP. Therefore, in this study, we examined morphological and functional changes in the retina using a chronic OIR model. Both the a- and b-waves in the OIR model recovered in a time-dependent manner at 4 weeks (w), 6 w, and 8 w, but the oscillatory potential (OP) amplitudes remained depressed following a return to normoxic conditions. Furthermore, decrease in the thicknesses of the inner plexiform layer (IPL) and inner nuclear layer (INL) at postnatal day (P) 17, 4 w, and 8 w and hyperpermeability of blood vessels were observed in conjunction with the decrease in the expression of claudin-5 and occludin at 8 w. The chronic OIR model revealed the following: (1) a decrease in OP amplitudes, (2) morphological abnormalities in the retinal cells (limited to the IPL and INL) and blood vessels, and (3) an increase in retinal vascular permeability via the impairment of the tight junction proteins. These findings suggest that the experimental animal model used in this study is suitable for elucidating the pathogenesis of ROP and may lead to the development of potential therapeutic agents for ROP treatment.
Conflict of interest statement
Figures
References
-
- Reisner DS, Hansen RM, Findl O, Petersen RA, Fulton AB. Dark-adapted thresholds in children with histories of mild retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1997;38:1175–1183. - PubMed
-
- Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol. 2001;119:499–505. - PubMed
-
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. - PubMed
-
- Chikaraishi Y, Shimazawa M, Hara H. New quantitative analysis, using high-resolution images, of oxygen-induced retinal neovascularization in mice. Exp Eye Res. 2007;84:529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

