Climate-based models for understanding and forecasting dengue epidemics
- PMID: 22348154
- PMCID: PMC3279338
- DOI: 10.1371/journal.pntd.0001470
Climate-based models for understanding and forecasting dengue epidemics
Abstract
Background: Dengue dynamics are driven by complex interactions between human-hosts, mosquito-vectors and viruses that are influenced by environmental and climatic factors. The objectives of this study were to analyze and model the relationships between climate, Aedes aegypti vectors and dengue outbreaks in Noumea (New Caledonia), and to provide an early warning system.
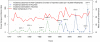
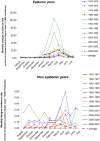
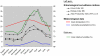
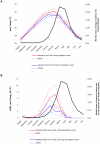
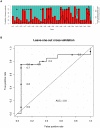
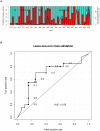
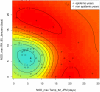
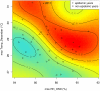
Methodology/principal findings: Epidemiological and meteorological data were analyzed from 1971 to 2010 in Noumea. Entomological surveillance indices were available from March 2000 to December 2009. During epidemic years, the distribution of dengue cases was highly seasonal. The epidemic peak (March-April) lagged the warmest temperature by 1-2 months and was in phase with maximum precipitations, relative humidity and entomological indices. Significant inter-annual correlations were observed between the risk of outbreak and summertime temperature, precipitations or relative humidity but not ENSO. Climate-based multivariate non-linear models were developed to estimate the yearly risk of dengue outbreak in Noumea. The best explicative meteorological variables were the number of days with maximal temperature exceeding 32°C during January-February-March and the number of days with maximal relative humidity exceeding 95% during January. The best predictive variables were the maximal temperature in December and maximal relative humidity during October-November-December of the previous year. For a probability of dengue outbreak above 65% in leave-one-out cross validation, the explicative model predicted 94% of the epidemic years and 79% of the non epidemic years, and the predictive model 79% and 65%, respectively.
Conclusions/significance: The epidemic dynamics of dengue in Noumea were essentially driven by climate during the last forty years. Specific conditions based on maximal temperature and relative humidity thresholds were determinant in outbreaks occurrence. Their persistence was also crucial. An operational model that will enable health authorities to anticipate the outbreak risk was successfully developed. Similar models may be developed to improve dengue management in other countries.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- WHO Dengue guidelines for diagnosis, treatment, prevention and control. 2009. Available: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. Accessed 10 December 2010. - PubMed
-
- Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, et al. Dengue and dengue haemorrhagic fever fever. Lancet. 1998;352:971–977. - PubMed
-
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economical problem in the 21th century. Trends Microbiol. 2002;10:100–103. - PubMed
-
- Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

