First clinical experience with the magnetic resonance imaging contrast agent and superoxide dismutase mimetic mangafodipir as an adjunct in cancer chemotherapy-a translational study
- PMID: 22348174
- PMCID: PMC3281413
- DOI: 10.1593/tlo.11277
First clinical experience with the magnetic resonance imaging contrast agent and superoxide dismutase mimetic mangafodipir as an adjunct in cancer chemotherapy-a translational study
Abstract
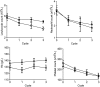
Preclinical research suggests that the clinically approved magnetic resonance imaging contrast agent mangafodipir may protect against adverse events (AEs) caused by chemotherapy, without interfering negatively with the anticancer efficacy. The present translational study tested if pretreatment with mangafodipir lowers AEs during curative (adjuvant) FOLFOX6 chemotherapy in stage III colon cancer (Dukes' C). The study was originally scheduled to include 20 patients, but because of the unforeseen withdrawal of mangafodipir from the market, the study had to be closed after 14 patients had been included. The withdrawal of mangafodipir was purely based on commercial considerations from the producer and not on any safety concerns. The patients were treated throughout the first 3 of 12 scheduled cycles. Patients were randomized to a 5-minute infusion of either mangafodipir or placebo (7 in each group). AEs were evaluated according to the National Cancer Institute's (NCI) Common Terminology Criteria for Adverse Events and the Sanofi-NCI criteria. The primary end points were neutropenia and neurosensory toxicity. There were four AEs of grade 3 (severe) and one AE of grade 4 (life threatening) in four patients in the placebo group, whereas there were none in the mangafodipir group (P < .05). Of the grade 3 and 4 events, two were neutropenia and one was neurosensory toxicity. Furthermore, white blood cell count was statistically, significantly higher in the mangafodipir group than in the placebo group (P < .01) after treatment with FOLFOX. This small feasibility study seems to confirm what has been demonstrated preclinically, namely, that pretreatment with mangafodipir lowers AEs during adjuvant 5-fluorouracil plus oxaliplatin-based chemotherapy in colon cancer patients.
Figures
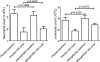
References
-
- O'Neil BH, Goldberg RM. Innovations in chemotherapy for metastatic colorectal cancer: an update of recent clinical trials. Oncologist. 2008;13:1074–1083. - PubMed
-
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. - PubMed
-
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- Asplund A, Grant D, Karlsson JOG. Mangafodipir (MnDPDP) and MnCl2-induced endothelium-dependent relaxation in bovine mesenteric arteries. J Pharmacol Exp Ther. 1994;271:609–618. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
