Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy
- PMID: 22348197
- PMCID: PMC3281420
- DOI: 10.3390/cancers3021639
Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy
Abstract
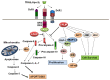
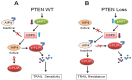
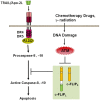
Cellular FLICE (FADD-like IL-1beta-converting enzyme)-inhibitory protein (c-FLIP) is a major resistance factor and critical anti-apoptotic regulator that inhibits tumor necrosis factor-alpha (TNF-alpha), Fas-L, and TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis as well as chemotherapy-triggered apoptosis in malignant cells. c-FLIP is expressed as long (c-FLIP(L)), short (c-FLIP(S)), and c-FLIP(R) splice variants in human cells. c-FLIP binds to FADD and/or caspase-8 or -10 in a ligand-dependent and-independent fashion, which in turn prevents death-inducing signaling complex (DISC) formation and subsequent activation of the caspase cascade. Moreover, c-FLIP(L) and c-FLIP(S) are known to have multifunctional roles in various signaling pathways, as well as activating and/or upregulating several cytoprotective signaling molecules. Upregulation of c-FLIP has been found in various tumor types, and its downregulation has been shown to restore apoptosis triggered by cytokines and various chemotherapeutic agents. Hence, c-FLIP is an important target for cancer therapy. For example, small interfering RNAs (siRNAs) that specifically knockdown the expression of c-FLIP(L) in diverse human cancer cell lines augmented TRAIL-induced DISC recruitment and increased the efficacy of chemotherapeutic agents, thereby enhancing effector caspase stimulation and apoptosis. Moreover, small molecules causing degradation of c-FLIP as well as decreasing mRNA and protein levels of c-FLIP(L) and c-FLIP(S) splice variants have been found, and efforts are underway to develop other c-FLIP-targeted cancer therapies. This review focuses on (1) the functional role of c-FLIP splice variants in preventing apoptosis and inducing cytokine and drug resistance; (2) the molecular mechanisms that regulate c-FLIP expression; and (3) strategies to inhibit c-FLIP expression and function.
Figures

Similar articles
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
-
Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy.Curr Cancer Drug Targets. 2008 Feb;8(1):37-46. doi: 10.2174/156800908783497087. Curr Cancer Drug Targets. 2008. PMID: 18288942 Free PMC article. Review.
-
Roles of c-FLIP in Apoptosis, Necroptosis, and Autophagy.J Carcinog Mutagen. 2013;Suppl 6:003. doi: 10.4172/2157-2518.S6-003. J Carcinog Mutagen. 2013. PMID: 25379355 Free PMC article.
-
Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis.J Biol Chem. 2005 May 13;280(19):19401-9. doi: 10.1074/jbc.M413962200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760909
-
Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5.J Biol Chem. 2004 Dec 31;279(53):55594-601. doi: 10.1074/jbc.M401056200. Epub 2004 Oct 14. J Biol Chem. 2004. PMID: 15485835 Free PMC article.
Cited by
-
A critical dose of doxorubicin is required to alter the gene expression profiles in MCF-7 cells acquiring multidrug resistance.PLoS One. 2015 Jan 30;10(1):e0116747. doi: 10.1371/journal.pone.0116747. eCollection 2015. PLoS One. 2015. PMID: 25635866 Free PMC article.
-
Epigenetic regulation of the TRAIL/Apo2L apoptotic pathway by histone deacetylase inhibitors: an attractive approach to bypass melanoma immunotherapy resistance.Am J Clin Exp Immunol. 2013 Feb 27;2(1):55-74. Print 2013. Am J Clin Exp Immunol. 2013. PMID: 23885325 Free PMC article.
-
c-FLIP, a Novel Biomarker for Cancer Prognosis, Immunosuppression, Alzheimer's Disease, Chronic Obstructive Pulmonary Disease (COPD), and a Rationale Therapeutic Target.Biomark J. 2019;5(1):4. doi: 10.36648/2472-1646.5.1.59. Epub 2019 Apr 26. Biomark J. 2019. PMID: 32352084 Free PMC article.
-
Ischemia-reperfusion injury of the retina is linked to necroptosis via the ERK1/2-RIP3 pathway.Mol Vis. 2014 Sep 24;20:1374-87. eCollection 2014. Mol Vis. 2014. PMID: 25352744 Free PMC article.
-
Anoikis and EMT: Lethal "Liaisons" during Cancer Progression.Crit Rev Oncog. 2016;21(3-4):155-168. doi: 10.1615/CritRevOncog.2016016955. Crit Rev Oncog. 2016. PMID: 27915969 Free PMC article. Review.
References
-
- Perez E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009;8:2086–2095. - PubMed
-
- Clarke R., Leonessa F., Trock B. Multidrug resistance/P-glycoprotein and breast cancer: Review and meta-analysis. Semin. Oncol. 2005;32:S9–S15. - PubMed
-
- Glavinas H., Krajcsi P., Cserepes J., Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004;1:27–42. - PubMed
-
- Roberti A., La Sala D., Cinti C. Multiple genetic and epigenetic interacting mechanisms contribute to clonally selection of drug-resistant tumors: Current views and new therapeutic prospective. J. Cell. Physiol. 2006;207:571–581. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
