Comprehensive behavioral analysis of ENU-induced Disc1-Q31L and -L100P mutant mice
- PMID: 22348257
- PMCID: PMC3392730
- DOI: 10.1186/1756-0500-5-108
Comprehensive behavioral analysis of ENU-induced Disc1-Q31L and -L100P mutant mice
Abstract
Background: Disrupted-in-Schizophrenia 1 (DISC1) is considered to be a candidate susceptibility gene for psychiatric disorders, including schizophrenia, bipolar disorder, and major depression. A recent study reported that N-ethyl-N-nitrosourea (ENU)-induced mutations in exon 2 of the mouse Disc1 gene, which resulted in the amino acid exchange of Q31L and L100P, caused an increase in depression-like behavior in 31 L mutant mice and schizophrenia-like behavior in 100P mutant mice; thus, these are potential animal models of psychiatric disorders. However, remaining heterozygous mutations that possibly occur in flanking genes other than Disc1 itself might induce behavioral abnormalities in the mutant mice. Here, to confirm the effects of Disc1-Q31L and Disc1-L100P mutations on behavioral phenotypes and to investigate the behaviors of the mutant mice in more detail, the mutant lines were backcrossed to C57BL/6JJcl through an additional two generations and the behaviors were analyzed using a comprehensive behavioral test battery.
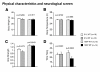
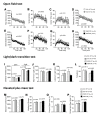
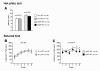
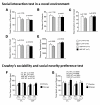
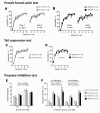
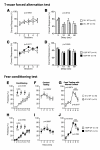
Results: Contrary to expectations, 31 L mutant mice showed no significant behavioral differences when compared with wild-type control mice in any of the behavioral tests, including the Porsolt forced swim and tail suspension tests, commonly used tests for depression-like behavior. Also, 100P mutant mice exhibited no differences in almost all of the behavioral tests, including the prepulse inhibition test for measuring sensorimotor gating, which is known to be impaired in schizophrenia patients; however, 100P mutant mice showed higher locomotor activity compared with wild-type control mice in the light/dark transition test.
Conclusions: Although these results are partially consistent with the previous study in that there was hyperactivity in 100P mutant mice, the vast majority of the results are inconsistent with those of the previous study; this discrepancy may be explained by differences in the genetic background of the mice, the laboratory environment, experimental protocols, and more. Further behavioral studies under various experimental conditions are necessary to determine whether these Disc1 mutant mouse lines are suitable animal models of schizophrenia and major depression.
Figures
References
-
- Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004;9:14–27. - PubMed
-
- Stefansson H, Petursson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. - DOI - PMC - PubMed
-
- Ekelund J, Lichtermann D, Hovatta I, Ellonen P, Suvisaari J, Terwilliger JD, Juvonen H, Varilo T, Arajärvi R, Kokko-Sahin ML, Lönnqvist J, Peltonen L. Genome-wide scan for schizophrenia in the Finnish population: evidence for a locus on chromosome 7q22. Hum Mol Genet. 2000;9:1049–1057. doi: 10.1093/hmg/9.7.1049. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

