Protein ligation in living cells using sortase
- PMID: 22348280
- PMCID: PMC3490390
- DOI: 10.1111/j.1600-0854.2012.01345.x
Protein ligation in living cells using sortase
Abstract
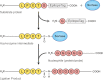
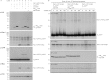
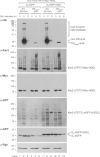
Sortagging is a versatile method for site-specific modification of proteins as applied to a variety of in vitro reactions. Here, we explore possibilities of adapting the sortase method for use in living cells. For intracellular sortagging, we employ the Ca²⁺-independent sortase A transpeptidase (SrtA) from Streptococcus pyogenes. Substrate proteins were equipped with the C-terminal sortase-recognition motif (LPXTG); we used proteins with an N-terminal (oligo)glycine as nucleophiles. We show that sortase-dependent protein ligation can be achieved in Saccharomyces cerevisiae and in mammalian HEK293T cells, both in the cytosol and in the lumen of the endoplasmic reticulum (ER). ER luminal sortagging enables secretion of the reaction products, among which circular polypeptides. Protein ligation of substrate and nucleophile occurs within 30 min of translation. The versatility of the method is shown by protein ligation of multiple substrates with green fluorescent protein-based nucleophiles in different intracellular compartments.
© 2012 John Wiley & Sons A/S.
Figures



References
-
- Popp MW, Ploegh HL. Making and breaking peptide bonds: protein engineering using sortase. Angew Chem Int Ed Engl. 2011;50:5024–5032. - PubMed
-
- Pallen MJ, Lam AC, Antonio M, Dunbar K. An embarrassment of sortases - a richness of substrates? Trends Microbiol. 2001;9:97–102. - PubMed
-
- Mao H, Hart SA, Schink A, Pollok BA. Sortase-mediated protein ligation: a new method for protein engineering. J Am Chem Soc. 2004;126:2670–2671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

