Six-month low level chlorine dioxide gas inhalation toxicity study with two-week recovery period in rats
- PMID: 22348507
- PMCID: PMC3298712
- DOI: 10.1186/1745-6673-7-2
Six-month low level chlorine dioxide gas inhalation toxicity study with two-week recovery period in rats
Abstract
Background: Chlorine dioxide (CD) gas has a potent antimicrobial activity at extremely low concentration and may serve as a new tool for infection control occupationally as well as publicly. However, it remains unknown whether the chronic exposure of CD gas concentration effective against microbes is safe. Therefore, long-term, low concentration CD gas inhalation toxicity was studied in rats as a six-month continuous whole-body exposure followed by a two-week recovery period, so as to prove that the CD gas exposed up to 0.1 ppm (volume ratio) is judged as safe on the basis of a battery of toxicological examinations.
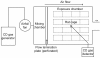
Methods: CD gas at 0.05 ppm or 0.1 ppm for 24 hours/day and 7 days/week was exposed to rats for 6 months under an unrestrained condition with free access to chow and water in a chamber so as to simulate the ordinary lifestyle in human. The control animals were exposed to air only. During the study period, the body weight as well as the food and water consumptions were recorded. After the 6-month exposure and the 2-week recovery period, animals were sacrificed and a battery of toxicological examinations, including biochemistry, hematology, necropsy, organ weights and histopathology, were performed.
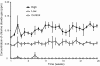
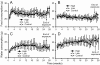
Results: Well regulated levels of CD gas were exposed throughout the chamber over the entire study period. No CD gas-related toxicity sign was observed during the whole study period. No significant difference was observed in body weight gain, food and water consumptions, and relative organ weight. In biochemistry and hematology examinations, changes did not appear to be related to CD gas toxicity. In necropsy and histopathology, no CD gas-related toxicity was observed even in expected target respiratory organs.
Conclusions: CD gas up to 0.1 ppm, exceeding the level effective against microbes, exposed to whole body in rats continuously for six months was not toxic, under a condition simulating the conventional lifestyle in human.
Figures

Similar articles
-
NTP Toxicology and Carcinogenesis Studies of Tetrafluoroethylene (CAS No. 116-14-3) in F344 Rats and B6C3F1 Mice (Inhalation Studies).Natl Toxicol Program Tech Rep Ser. 1997 Apr;450:1-321. Natl Toxicol Program Tech Rep Ser. 1997. PMID: 12594525
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
NTP Toxicology and Carcinogenesis Studies of Isobutyraldehyde (CAS No. 78-84-2) in F344/N Rats and B6C3F1 Mice (Inhalation Studies).Natl Toxicol Program Tech Rep Ser. 1999 Feb;472:1-242. Natl Toxicol Program Tech Rep Ser. 1999. PMID: 12579201
-
NTP technical report on the toxicity studies of Isoprene (CAS No. 78-79-5) Administered by Inhalation to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Jan;31:1-G5. Toxic Rep Ser. 1995. PMID: 12209193
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
Cited by
-
Effects of continuous exposure to low concentration of ClO2 gas on the growth, viability, and maintenance of undifferentiated MSCs in long-term cultures.Regen Ther. 2020 Feb 24;14:184-190. doi: 10.1016/j.reth.2019.12.007. eCollection 2020 Jun. Regen Ther. 2020. PMID: 32128355 Free PMC article.
-
A review of methods to reduce the probability of the airborne spread of COVID-19 in ventilation systems and enclosed spaces.Environ Res. 2022 Jan;203:111765. doi: 10.1016/j.envres.2021.111765. Epub 2021 Jul 28. Environ Res. 2022. PMID: 34331921 Free PMC article. Review.
-
Acute oral toxicity and mucosal irritation of a mouthwash containing chlorhexidine and chlorine dioxide in animal models.J Oral Biol Craniofac Res. 2025 Jul-Aug;15(4):770-774. doi: 10.1016/j.jobcr.2025.04.013. Epub 2025 May 17. J Oral Biol Craniofac Res. 2025. PMID: 40487357 Free PMC article.
-
Effect of extremely low-concentration gaseous chlorine dioxide against surface Escherichia coli, Pseudomonas aeruginosa and Acinetobacter baumannii in wet conditions on glass dishes.BMC Res Notes. 2020 Feb 12;13(1):69. doi: 10.1186/s13104-020-4925-5. BMC Res Notes. 2020. PMID: 32051032 Free PMC article.
-
Formulation of Chlorine-Dioxide-Releasing Nanofibers for Disinfection in Humid and CO2-Rich Environment.Nanomaterials (Basel). 2022 Apr 27;12(9):1481. doi: 10.3390/nano12091481. Nanomaterials (Basel). 2022. PMID: 35564190 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

