The embryonic leaf identity gene FUSCA3 regulates vegetative phase transitions by negatively modulating ethylene-regulated gene expression in Arabidopsis
- PMID: 22348746
- PMCID: PMC3305478
- DOI: 10.1186/1741-7007-10-8
The embryonic leaf identity gene FUSCA3 regulates vegetative phase transitions by negatively modulating ethylene-regulated gene expression in Arabidopsis
Abstract
Background: The embryonic temporal regulator FUSCA3 (FUS3) plays major roles in the establishment of embryonic leaf identity and the regulation of developmental timing. Loss-of-function mutations of this B3 domain transcription factor result in replacement of cotyledons with leaves and precocious germination, whereas constitutive misexpression causes the conversion of leaves into cotyledon-like organs and delays vegetative and reproductive phase transitions.
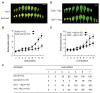
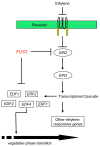
Results: Herein we show that activation of FUS3 after germination dampens the expression of genes involved in the biosynthesis and response to the plant hormone ethylene, whereas a loss-of-function fus3 mutant shows many phenotypes consistent with increased ethylene signaling. This FUS3-dependent regulation of ethylene signaling also impinges on timing functions outside embryogenesis. Loss of FUS3 function results in accelerated vegetative phase change, and this is again partially dependent on functional ethylene signaling. This alteration in vegetative phase transition is dependent on both embryonic and vegetative FUS3 function, suggesting that this important transcriptional regulator controls both embryonic and vegetative developmental timing.
Conclusion: The results of this study indicate that the embryonic regulator FUS3 not only controls the embryonic-to-vegetative phase transition through hormonal (ABA/GA) regulation but also functions postembryonically to delay vegetative phase transitions by negatively modulating ethylene-regulated gene expression.
Figures
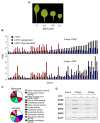
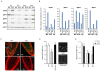
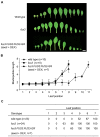
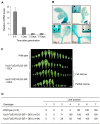
Comment in
-
Ethylene and the regulation of plant development.BMC Biol. 2012 Feb 20;10:9. doi: 10.1186/1741-7007-10-9. BMC Biol. 2012. PMID: 22348804 Free PMC article.
Similar articles
-
The C-terminal domain of FUSCA3 negatively regulates mRNA and protein levels, and mediates sensitivity to the hormones abscisic acid and gibberellic acid in Arabidopsis.Plant J. 2010 Oct;64(1):100-13. doi: 10.1111/j.1365-313X.2010.04307.x. Epub 2010 Aug 31. Plant J. 2010. PMID: 20663088
-
AKIN10 and FUSCA3 interact to control lateral organ development and phase transitions in Arabidopsis.Plant J. 2012 Mar;69(5):809-21. doi: 10.1111/j.1365-313X.2011.04832.x. Epub 2011 Dec 15. Plant J. 2012. PMID: 22026387
-
Repression of the LEAFY COTYLEDON 1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE 3-LIKE B3 genes.Plant Physiol. 2007 Feb;143(2):902-11. doi: 10.1104/pp.106.092320. Epub 2006 Dec 8. Plant Physiol. 2007. PMID: 17158584 Free PMC article.
-
Ethylene and Hormonal Cross Talk in Vegetative Growth and Development.Plant Physiol. 2015 Sep;169(1):61-72. doi: 10.1104/pp.15.00724. Epub 2015 Jul 31. Plant Physiol. 2015. PMID: 26232489 Free PMC article. Review.
-
Ethylene-mediated regulation of coleoptile elongation in rice seedlings.Plant Cell Environ. 2023 Apr;46(4):1060-1074. doi: 10.1111/pce.14492. Epub 2022 Dec 2. Plant Cell Environ. 2023. PMID: 36397123 Review.
Cited by
-
Ectopic overexpression of castor bean LEAFY COTYLEDON2 (LEC2) in Arabidopsis triggers the expression of genes that encode regulators of seed maturation and oil body proteins in vegetative tissues.FEBS Open Bio. 2013 Nov 23;4:25-32. doi: 10.1016/j.fob.2013.11.003. eCollection 2013. FEBS Open Bio. 2013. PMID: 24363987 Free PMC article.
-
Juvenile Leaves or Adult Leaves: Determinants for Vegetative Phase Change in Flowering Plants.Int J Mol Sci. 2020 Dec 21;21(24):9753. doi: 10.3390/ijms21249753. Int J Mol Sci. 2020. PMID: 33371265 Free PMC article. Review.
-
FOREVER YOUNG FLOWER Negatively Regulates Ethylene Response DNA-Binding Factors by Activating an Ethylene-Responsive Factor to Control Arabidopsis Floral Organ Senescence and Abscission.Plant Physiol. 2015 Aug;168(4):1666-83. doi: 10.1104/pp.15.00433. Epub 2015 Jun 10. Plant Physiol. 2015. PMID: 26063506 Free PMC article.
-
Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid-ethylene antagonism mediated by histone deacetylation.Plant Cell. 2013 Jan;25(1):149-66. doi: 10.1105/tpc.112.108191. Epub 2013 Jan 31. Plant Cell. 2013. PMID: 23371947 Free PMC article.
-
Functional Analysis of the Arabidopsis TETRASPANIN Gene Family in Plant Growth and Development.Plant Physiol. 2015 Nov;169(3):2200-14. doi: 10.1104/pp.15.01310. Epub 2015 Sep 28. Plant Physiol. 2015. PMID: 26417009 Free PMC article.
References
-
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. - PubMed
-
- Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–465. - PubMed
-
- Kerstetter RA, Poethig RS. The specification of leaf identity during shoot development. Annu Rev Cell Dev Biol. 1998;14:373–398. - PubMed
-
- Henderson IR, Dean C. Control of Arabidopsis flowering: the chill before the bloom. Development. 2004;131:3829–3838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

