TOPS: an internet-based system to prevent healthy subjects from over-volunteering for clinical trials
- PMID: 22349161
- PMCID: PMC3374109
- DOI: 10.1007/s00228-012-1231-8
TOPS: an internet-based system to prevent healthy subjects from over-volunteering for clinical trials
Abstract
Aim: Our aim was to set up a system to help UK clinical research units to prevent healthy volunteers from participating in more than one non-therapeutic trial simultaneously, or from starting a second trial too soon after the first.
Methods: TOPS (The Over-volunteering Prevention System) is internet-based, simple and quick to use, free to users and a charity run by a Board of Trustees. Users enter only two or three pieces of information: (1) 'National Insurance number' (NINO) of UK citizens, or 'passport number' and country of origin of non-UK citizens, as their identifier, (2) 'date of last dose' of trial medicine or (3) 'never dosed'. Subjects must consent, but TOPS collects only non-personal data, so it does not require Ethics Committee approval and is not covered by the Data Protection Act.
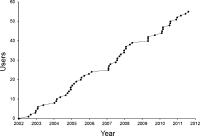
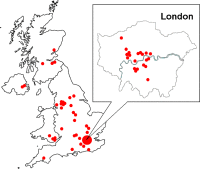
Results: A total of 55 research units (29 clinical research organisations, 5 pharmaceutical companies, 13 universities and 8 hospitals) throughout the UK have registered to use TOPS, and have entered 124,906 volunteers since we launched it. All commercial and many non-commercial units now use TOPS. In our unit, no subject has to the best of our knowledge participated in two trials simultaneously. TOPS has reduced to <1% the incidence of subjects attempting to volunteer within 3 months of completing another trial elsewhere, and very few have to our knowledge succeeded.
Conclusion: TOPS is widely used and effective, and helps research units to comply with UK clinical trial regulations.
Conflict of interest statement
HMR funds TOPS. All authors are employees of HMR. MB and JK are Trustees of TOPS, a registered charity (No. 1100989).
Figures
Comment in
-
A pan-European registration system for volunteer participation is within sight.Eur J Clin Pharmacol. 2013 Mar;69(3):729. doi: 10.1007/s00228-012-1349-8. Epub 2012 Jul 29. Eur J Clin Pharmacol. 2013. PMID: 22843015 No abstract available.
-
TOPS: an internet-based system to prevent healthy subjects from over-volunteering for clinical trials.Eur J Clin Pharmacol. 2013 Mar;69(3):731. doi: 10.1007/s00228-012-1351-1. Epub 2012 Sep 12. Eur J Clin Pharmacol. 2013. PMID: 22968810 No abstract available.
References
-
- Royal College of Physicians (1986) Research on healthy volunteers. A report of the Royal College of Physicians. J R Coll Physicians 20:3–17 - PubMed
-
- Association of theBritish Pharmaceutical Industry (2007) Guidelines for phase 1 clinical trials. Association of the British Pharmaceutical Industry, London
-
- Food and Drug Administration (FDA) (2000) Good clinical practice regulations. Code of Federal Regulations 21 CFR Part 56.110. FDA, Washington D.C.
-
- Boyce M, Nentwich H, Melbourne W, Warrington S. TOPS: the over-volunteering prevention system. Br J Clin Pharmacol. 2003;55:643P. doi: 10.1046/j.1365-2125.2003.01923.x. - DOI
-
- National Science and Technology Council (2006) Fingerprint recognition. Updated 7 August 2006. Available at: http://www.biometrics.gov/Documents/fingerprintrec.pdf. Accessed 17 Aug 2011
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical