Identification of functional differences between recombinant human α and β cardiac myosin motors
- PMID: 22349210
- PMCID: PMC3375423
- DOI: 10.1007/s00018-012-0927-3
Identification of functional differences between recombinant human α and β cardiac myosin motors
Erratum in
-
Erratum to: Identification of functional differences between recombinant human α and β cardiac myosin motors.Cell Mol Life Sci. 2012 Dec;69(24):4239-55. doi: 10.1007/s00018-012-1111-5. Cell Mol Life Sci. 2012. PMID: 23001010 Free PMC article.
Abstract
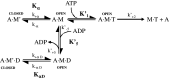
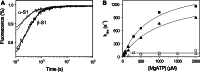
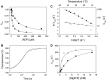
The myosin isoform composition of the heart is dynamic in health and disease and has been shown to affect contractile velocity and force generation. While different mammalian species express different proportions of α and β myosin heavy chain, healthy human heart ventricles express these isoforms in a ratio of about 1:9 (α:β) while failing human ventricles express no detectable α-myosin. We report here fast-kinetic analysis of recombinant human α and β myosin heavy chain motor domains. This represents the first such analysis of any human muscle myosin motor and the first of α-myosin from any species. Our findings reveal substantial isoform differences in individual kinetic parameters, overall contractile character, and predicted cycle times. For these parameters, α-subfragment 1 (S1) is far more similar to adult fast skeletal muscle myosin isoforms than to the slow β isoform despite 91% sequence identity between the motor domains of α- and β-myosin. Among the features that differentiate α- from β-S1: the ATP hydrolysis step of α-S1 is ~ten-fold faster than β-S1, α-S1 exhibits ~five-fold weaker actin affinity than β-S1, and actin·α-S1 exhibits rapid ADP release, which is >ten-fold faster than ADP release for β-S1. Overall, the cycle times are ten-fold faster for α-S1 but the portion of time each myosin spends tightly bound to actin (the duty ratio) is similar. Sequence analysis points to regions that might underlie the basis for this finding.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Erratum to: Identification of functional differences between recombinant human α and β cardiac myosin motors.Cell Mol Life Sci. 2012 Dec;69(24):4239-55. doi: 10.1007/s00018-012-1111-5. Cell Mol Life Sci. 2012. PMID: 23001010 Free PMC article.
-
Transgenic mouse α- and β-cardiac myosins containing the R403Q mutation show isoform-dependent transient kinetic differences.J Biol Chem. 2013 May 24;288(21):14780-7. doi: 10.1074/jbc.M113.450668. Epub 2013 Apr 11. J Biol Chem. 2013. PMID: 23580644 Free PMC article.
-
Kinetic mechanism of Nicotiana tabacum myosin-11 defines a new type of a processive motor.FASEB J. 2015 Jan;29(1):81-94. doi: 10.1096/fj.14-254763. Epub 2014 Oct 17. FASEB J. 2015. PMID: 25326536
-
Myosin II isoforms in smooth muscle: heterogeneity and function.Am J Physiol Cell Physiol. 2007 Aug;293(2):C493-508. doi: 10.1152/ajpcell.00131.2007. Epub 2007 May 2. Am J Physiol Cell Physiol. 2007. PMID: 17475667 Review.
-
Modulation of cardiac performance by motor protein gene transfer.Ann N Y Acad Sci. 2008 Mar;1123:96-104. doi: 10.1196/annals.1420.011. Ann N Y Acad Sci. 2008. PMID: 18375581 Review.
Cited by
-
Hypertrophic cardiomyopathy: Mutations to mechanisms to therapies.Front Physiol. 2022 Sep 26;13:975076. doi: 10.3389/fphys.2022.975076. eCollection 2022. Front Physiol. 2022. PMID: 36225299 Free PMC article. Review.
-
Cardiac myosin contraction and mechanotransduction in health and disease.J Biol Chem. 2021 Nov;297(5):101297. doi: 10.1016/j.jbc.2021.101297. Epub 2021 Oct 9. J Biol Chem. 2021. PMID: 34634306 Free PMC article. Review.
-
Electrostatic interaction of loop 1 and backbone of human cardiac myosin regulates the rate of ATP induced actomyosin dissociation.J Muscle Res Cell Motil. 2022 Mar;43(1):1-8. doi: 10.1007/s10974-021-09611-z. Epub 2021 Nov 26. J Muscle Res Cell Motil. 2022. PMID: 34825297 Free PMC article.
-
Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases.Biophys J. 2014 Mar 18;106(6):1236-49. doi: 10.1016/j.bpj.2014.02.011. Biophys J. 2014. PMID: 24655499 Free PMC article. Review.
-
Hypothesis and theory: mechanical instabilities and non-uniformities in hereditary sarcomere myopathies.Front Physiol. 2014 Sep 15;5:350. doi: 10.3389/fphys.2014.00350. eCollection 2014. Front Physiol. 2014. PMID: 25309450 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

