Cytoskeleton responses in wound repair
- PMID: 22349211
- PMCID: PMC3388155
- DOI: 10.1007/s00018-012-0928-2
Cytoskeleton responses in wound repair
Abstract
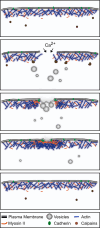
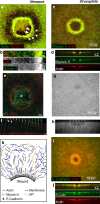
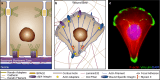
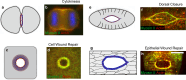
Wound repair on the cellular and multicellular levels is essential to the survival of complex organisms. In order to avoid further damage, prevent infection, and restore normal function, cells and tissues must rapidly seal and remodel the wounded area. The cytoskeleton is an important component of wound repair in that it is needed for actomyosin contraction, recruitment of repair machineries, and cell migration. Recent use of model systems and high-resolution microscopy has provided new insight into molecular aspects of the cytoskeletal response during wound repair. Here we discuss the role of the cytoskeleton in single-cell, embryonic, and adult repair, as well as the striking resemblance of these processes to normal developmental events and many diseases.
Figures





Similar articles
-
Into the breach: how cells cope with wounds.Open Biol. 2018 Oct 3;8(10):180135. doi: 10.1098/rsob.180135. Open Biol. 2018. PMID: 30282661 Free PMC article. Review.
-
Wound repair: toward understanding and integration of single-cell and multicellular wound responses.Annu Rev Cell Dev Biol. 2011;27:237-63. doi: 10.1146/annurev-cellbio-092910-154251. Epub 2011 Jun 20. Annu Rev Cell Dev Biol. 2011. PMID: 21721944 Free PMC article. Review.
-
Oxidative Stress Orchestrates Cell Polarity to Promote Embryonic Wound Healing.Dev Cell. 2018 Nov 5;47(3):377-387.e4. doi: 10.1016/j.devcel.2018.10.013. Dev Cell. 2018. PMID: 30399336
-
Parallels between tissue repair and embryo morphogenesis.Development. 2004 Jul;131(13):3021-34. doi: 10.1242/dev.01253. Development. 2004. PMID: 15197160 Review.
-
Forceful closure: cytoskeletal networks in embryonic wound repair.Mol Biol Cell. 2019 Jun 1;30(12):1353-1358. doi: 10.1091/mbc.E18-04-0248. Mol Biol Cell. 2019. PMID: 31145669 Free PMC article. Review.
Cited by
-
Aerobic glycolysis is important for zebrafish larval wound closure and tail regeneration.Wound Repair Regen. 2022 Nov;30(6):665-680. doi: 10.1111/wrr.13050. Epub 2022 Oct 5. Wound Repair Regen. 2022. PMID: 36148505 Free PMC article.
-
One-Week Dynamic Changes in Cardiac Proteomes After Cardiac Radioablation in Experimental Rat Model.Front Cardiovasc Med. 2022 Jun 28;9:898222. doi: 10.3389/fcvm.2022.898222. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35837601 Free PMC article.
-
The Micro-RNA Cargo of Extracellular Vesicles Released by Human Adipose Tissue-Derived Mesenchymal Stem Cells Is Modified by Obesity.Front Cell Dev Biol. 2021 May 20;9:660851. doi: 10.3389/fcell.2021.660851. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095124 Free PMC article.
-
Negative air ions through the action of antioxidation, anti-inflammation, anti-apoptosis and angiogenesis ameliorate lipopolysaccharide induced acute lung injury and promote diabetic wound healing in rat.PLoS One. 2022 Oct 26;17(10):e0275748. doi: 10.1371/journal.pone.0275748. eCollection 2022. PLoS One. 2022. PMID: 36288391 Free PMC article.
-
Into the breach: how cells cope with wounds.Open Biol. 2018 Oct 3;8(10):180135. doi: 10.1098/rsob.180135. Open Biol. 2018. PMID: 30282661 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

