Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings
- PMID: 22349459
- PMCID: PMC3343656
- DOI: 10.1038/cr.2012.29
Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings
Abstract
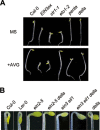
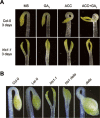
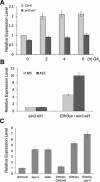
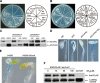
Dark-grown Arabidopsis seedlings develop an apical hook when germinating in soil, which protects the cotyledons and apical meristematic tissues when protruding through the soil. Several hormones are reported to distinctly modulate this process. Previous studies have shown that ethylene and gibberellins (GAs) coordinately regulate the hook development, although the underlying molecular mechanism is largely unknown. Here we showed that GA(3) enhanced while paclobutrazol repressed ethylene- and EIN3-overexpression (EIN3ox)-induced hook curvature, and della mutant exhibited exaggerated hook curvature, which required an intact ethylene signaling pathway. Genetic study revealed that GA-enhanced hook development was dependent on HOOKLESS 1 (HLS1), a central regulator mediating the input of the multiple signaling pathways during apical hook development. We further found that GA(3) induced (and DELLA proteins repressed) HLS1 expression in an ETHYLENE INSENSITIVE 3/EIN3-LIKE 1 (EIN3/EIL1)-dependent manner, whereby EIN3/EIL1 activated HLS1 transcription by directly binding to its promoter. Additionally, DELLA proteins were found to interact with the DNA-binding domains of EIN3/EIL1 and repress EIN3/EIL1-regulated HLS1 expression. Treatment with naphthylphthalamic acid, a polar auxin transport inhibitor, repressed the constitutively exaggerated hook curvature of EIN3ox line and della mutant, supporting that auxin functions downstream of the ethylene and GA pathways in hook development. Taken together, our results identify EIN3/EIL1 as a new class of DELLA-associated transcription factors and demonstrate that GA promotes apical hook formation in cooperation with ethylene partly by inducing the expression of HLS1 via derepression of EIN3/EIL1 functions.
Figures




References
-
- Darwin C, Darwin F.The power of movement in plantsNew York: D Appleton and Co, 188187–94.
-
- Silk WK, Erickson RO. Kinematics of hypocotyl curvature. Am J Bot. 1978;65:310–319.
-
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell. 2004;7:193–204. - PubMed
-
- Schwark A, Schierle J. Interaction of ethylene and auxin in the regulation of hook growth I: the role of auxin in different growing regions of the hypocotyl hook of Phaseolus vulgaris. J Plant Physiol. 1992;140:562–570.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

