Bilirubin oxidase from Magnaporthe oryzae: an attractive new enzyme for biotechnological applications
- PMID: 22350257
- PMCID: PMC3740731
- DOI: 10.1007/s00253-012-3926-2
Bilirubin oxidase from Magnaporthe oryzae: an attractive new enzyme for biotechnological applications
Abstract
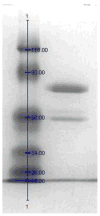
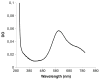
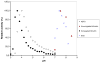
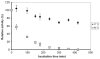
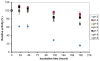
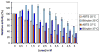
A novel bilirubin oxidase (BOD), from the rice blast fungus Magnaporthe oryzae, has been identified and isolated. The 64-kDa protein containing four coppers was successfully overexpressed in Pichia pastoris and purified to homogeneity in one step. Protein yield is more than 100 mg for 2 L culture, twice that of Myrothecium verrucaria. The k(cat)/K(m) ratio for conjugated bilirubin (1,513 mM⁻¹ s⁻¹) is higher than that obtained for the BOD from M. verrucaria expressed in native fungus (980 mM⁻¹ s⁻¹), with the lowest K(m) measured for any BOD highly desirable for detection of bilirubin in medical samples. In addition, this protein exhibits a half-life for deactivation >300 min at 37 °C, high stability at pH 7, and high tolerance towards urea, making it an ideal candidate for the elaboration of biofuel cells, powering implantable medical devices. Finally, this new BOD is efficient in decolorizing textile dyes such as Remazol brilliant Blue R, making it useful for environmentally friendly industrial applications.
Figures


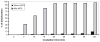
Similar articles
-
Purification, characterization and decolorization of bilirubin oxidase from Myrothecium verrucaria 3.2190.Fungal Biol. 2012 Aug;116(8):863-71. doi: 10.1016/j.funbio.2012.05.003. Epub 2012 Jun 1. Fungal Biol. 2012. PMID: 22862914
-
Bilirubin oxidase expression and activity enhancement from Myrothecium verrucaria in Aspergillus species.J Biosci Bioeng. 2024 Sep;138(3):212-217. doi: 10.1016/j.jbiosc.2024.06.002. Epub 2024 Jul 4. J Biosci Bioeng. 2024. PMID: 38969547
-
Bilirubin oxidase from Bacillus pumilus: a promising enzyme for the elaboration of efficient cathodes in biofuel cells.Biosens Bioelectron. 2012 May 15;35(1):140-146. doi: 10.1016/j.bios.2012.02.033. Epub 2012 Feb 25. Biosens Bioelectron. 2012. PMID: 22410485 Free PMC article.
-
Features and applications of bilirubin oxidases.Appl Microbiol Biotechnol. 2012 Oct;96(2):301-7. doi: 10.1007/s00253-012-4312-9. Epub 2012 Aug 3. Appl Microbiol Biotechnol. 2012. PMID: 22878843 Review.
-
Bilirubin oxidases in bioelectrochemistry: features and recent findings.Biosens Bioelectron. 2013 Dec 15;50:478-85. doi: 10.1016/j.bios.2013.07.014. Epub 2013 Jul 16. Biosens Bioelectron. 2013. PMID: 23911663 Review.
Cited by
-
The haustorial transcriptome of the cucurbit pathogen Podosphaera xanthii reveals new insights into the biotrophy and pathogenesis of powdery mildew fungi.BMC Genomics. 2019 Jul 4;20(1):543. doi: 10.1186/s12864-019-5938-0. BMC Genomics. 2019. PMID: 31272366 Free PMC article.
-
Spectroscopic and crystallographic characterization of "alternative resting" and "resting oxidized" enzyme forms of bilirubin oxidase: implications for activity and electrochemical behavior of multicopper oxidases.J Am Chem Soc. 2012 Mar 28;134(12):5548-51. doi: 10.1021/ja211872j. Epub 2012 Mar 21. J Am Chem Soc. 2012. PMID: 22413777 Free PMC article.
-
Fabricating high-purity graphite disk electrodes as a cost-effective alternative in fundamental electrochemistry research.Sci Rep. 2024 Feb 21;14(1):4258. doi: 10.1038/s41598-024-54654-0. Sci Rep. 2024. PMID: 38383697 Free PMC article.
-
Halides inhibition of multicopper oxidases studied by FTIR spectroelectrochemistry using azide as an active infrared probe.J Biol Inorg Chem. 2017 Dec;22(8):1179-1186. doi: 10.1007/s00775-017-1494-8. Epub 2017 Oct 3. J Biol Inorg Chem. 2017. PMID: 28975410
-
Enzymatic Fuel Cells: Towards Self-Powered Implantable and Wearable Diagnostics.Biosensors (Basel). 2018 Jan 29;8(1):11. doi: 10.3390/bios8010011. Biosensors (Basel). 2018. PMID: 29382147 Free PMC article. Review.
References
-
- Baldrian P. Fungal laccases—occurrence and properties. FEMS Microbiol Rev. 2006;30:212–242. - PubMed
-
- Barton SC, Gallaway J, Atanassov P. Enzymatic biofuel cells for implantable and microscale devices. Chem Rev. 2004;104:4867–4886. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brissos V, Pereira L, Munteanu FD, Cavaco-Paulo A, Martins LO. Expression system of CotA-laccase for directed evolution and high-throughput screenings for the oxidation of highredox potential dyes. Biotechnol J. 2009;4(4):558–563. - PubMed
-
- Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev. 2000;24 (1):45–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

