Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells
- PMID: 22350410
- PMCID: PMC3319847
- DOI: 10.1158/0008-5472.CAN-11-3132
Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells
Abstract
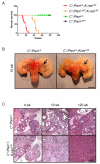
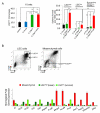
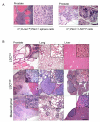
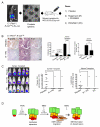
PTEN loss or PI3K/AKT signaling pathway activation correlates with human prostate cancer progression and metastasis. However, in preclinical murine models, deletion of Pten alone fails to mimic the significant metastatic burden that frequently accompanies the end stage of human disease. To identify additional pathway alterations that cooperate with PTEN loss in prostate cancer progression, we surveyed human prostate cancer tissue microarrays and found that the RAS/MAPK pathway is significantly elevated in both primary and metastatic lesions. In an attempt to model this event, we crossed conditional activatable K-ras(G12D/WT) mice with the prostate conditional Pten deletion model. Although RAS activation alone cannot initiate prostate cancer development, it significantly accelerated progression caused by PTEN loss, accompanied by epithelial-to-mesenchymal transition (EMT) and macrometastasis with 100% penetrance. A novel stem/progenitor subpopulation with mesenchymal characteristics was isolated from the compound mutant prostates, which was highly metastatic upon orthotopic transplantation. Importantly, inhibition of RAS/MAPK signaling by PD325901, a mitogen-activated protein (MAP)-extracellular signal-regulated (ER) kinase (MEK) inhibitor, significantly reduced the metastatic progression initiated from transplanted stem/progenitor cells. Collectively, our findings indicate that activation of RAS/MAPK signaling serves as a potentiating second hit to alteration of the PTEN/PI3K/AKT axis, and cotargeting both the pathways is highly effective in preventing the development of metastatic prostate cancers.
©2012 AACR.
Figures



References
-
- ACS American Cancer Society. Cancer Facts & Figures. 2010
-
- Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21. - PubMed
-
- Ma X, Ziel-van der Made AC, Autar B, et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

