RORα suppresses breast tumor invasion by inducing SEMA3F expression
- PMID: 22350413
- PMCID: PMC3319846
- DOI: 10.1158/0008-5472.CAN-11-2762
RORα suppresses breast tumor invasion by inducing SEMA3F expression
Abstract
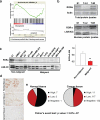
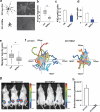
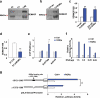
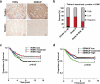
Inactivation of tumor suppressors and inhibitory microenvironmental factors is necessary for breast cancer invasion; therefore, identifying those suppressors and factors is crucial not only to advancing our knowledge of breast cancer, but also to discovering potential therapeutic targets. By analyzing gene expression profiles of polarized and disorganized human mammary epithelial cells in a physiologically relevant three-dimensional (3D) culture system, we identified retinoid orphan nuclear receptor alpha (RORα) as a transcription regulator of semaphorin 3F (SEMA3F), a suppressive microenvironmental factor. We showed that expression of RORα was downregulated in human breast cancer tissue and cell lines, and that reduced mRNA levels of RORα and SEMA3F correlated with poor prognosis. Restoring RORα expression reprogrammed breast cancer cells to form noninvasiveness structures in 3D culture and inhibited tumor growth in nude mice, accompanied by enhanced SEMA3F expression. Inactivation of RORα in nonmalignant human mammary epithelial cells inhibited SEMA3F transcription and impaired polarized acinar morphogenesis. Using chromatin immunoprecipitation and luciferase reporter assays, we showed that transcription of SEMA3F is directly regulated by RORα. Knockdown of SEMA3F in RORα-expressing cancer cells rescued the aggressive 3D phenotypes and tumor invasion. These findings indicate that RORα is a potential tumor suppressor and inhibits tumor invasion by inducing suppressive cell microenvironment.
©2012 AACR.
Figures



Similar articles
-
RORα, a potential tumor suppressor and therapeutic target of breast cancer.Int J Mol Sci. 2012 Nov 26;13(12):15755-66. doi: 10.3390/ijms131215755. Int J Mol Sci. 2012. PMID: 23443091 Free PMC article.
-
Retinoid orphan nuclear receptor alpha (RORα) suppresses the epithelial-mesenchymal transition (EMT) by directly repressing Snail transcription.J Biol Chem. 2022 Jul;298(7):102059. doi: 10.1016/j.jbc.2022.102059. Epub 2022 May 20. J Biol Chem. 2022. PMID: 35605663 Free PMC article.
-
Semaphorin 3F inhibits breast cancer metastasis by regulating the Akt-mTOR and TGFβ signaling pathways via neuropilin-2.Sci Rep. 2025 Mar 3;15(1):7394. doi: 10.1038/s41598-025-91559-y. Sci Rep. 2025. PMID: 40033046 Free PMC article.
-
RORalpha, a key to the development and functioning of the brain.Cerebellum. 2012 Jun;11(2):451-2. doi: 10.1007/s12311-011-0339-1. Cerebellum. 2012. PMID: 22223133 Review.
-
RAR-related orphan receptor alpha and the staggerer mice: a fine molecular story.Front Endocrinol (Lausanne). 2024 May 3;14:1300729. doi: 10.3389/fendo.2023.1300729. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38766309 Free PMC article. Review.
Cited by
-
RORα, a potential tumor suppressor and therapeutic target of breast cancer.Int J Mol Sci. 2012 Nov 26;13(12):15755-66. doi: 10.3390/ijms131215755. Int J Mol Sci. 2012. PMID: 23443091 Free PMC article.
-
Regulation of RORα Stability through PRMT5-Dependent Symmetric Dimethylation.Cancers (Basel). 2024 May 17;16(10):1914. doi: 10.3390/cancers16101914. Cancers (Basel). 2024. PMID: 38791992 Free PMC article.
-
Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORα.Front Vet Sci. 2023 Jul 10;10:1203302. doi: 10.3389/fvets.2023.1203302. eCollection 2023. Front Vet Sci. 2023. PMID: 37520005 Free PMC article.
-
β-adrenergic receptor-dependent alterations in murine cardiac transcript expression are differentially regulated by gefitinib in vivo.PLoS One. 2014 Jun 5;9(6):e99195. doi: 10.1371/journal.pone.0099195. eCollection 2014. PLoS One. 2014. PMID: 24901703 Free PMC article.
-
AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric cancer cells.Mol Cell Biochem. 2016 Jan;411(1-2):299-305. doi: 10.1007/s11010-015-2592-y. Epub 2015 Oct 24. Mol Cell Biochem. 2016. PMID: 26497305
References
-
- Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–47. - PubMed
-
- Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-Andre M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol. 1994;8:757–70. - PubMed
-
- Dussault I, Fawcett D, Matthyssen A, Bader JA, Giguere V. Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech Dev. 1998;70:147–53. - PubMed
-
- Matysiak-Scholze U, Nehls M. The structural integrity of ROR alpha isoforms is mutated in staggerer mice: cerebellar coexpression of ROR alpha1 and ROR alpha4. Genomics. 1997;43:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

