Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus)
- PMID: 22351487
- PMCID: PMC3336943
- DOI: 10.1093/aob/mcs024
Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus)
Abstract
Background and aims: Formation of cluster roots is one of the most specific root adaptations to nutrient deficiency. In white lupin (Lupinus albus), cluster roots can be induced by phosphorus (P) or iron (Fe) deficiency. The aim of the present work was to investigate the potential shared signalling pathway in P- and Fe-deficiency-induced cluster root formation.
Methods: Measurements were made of the internal concentration of nutrients, levels of nitric oxide (NO), citrate exudation and expression of some specific genes under four P × Fe combinations, namely (1) 50 µm P and 10 µm Fe (+P + Fe); (2) 0 P and 10 µm Fe (-P + Fe); (3) 50 µm P and 0 Fe (+P-Fe); and (4) 0 P and 0 Fe (-P-Fe), and these were examined in relation to the formation of cluster roots.
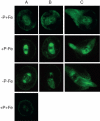
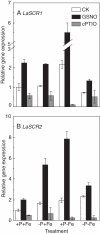
Key results: The deficiency of P, Fe or both increased the cluster root number and cluster zones. It also enhanced NO accumulation in pericycle cells and rootlet primordia at various stages of cluster root development. The formation of cluster roots and rootlet primordia, together with the expression of LaSCR1 and LaSCR2 which is crucial in cluster root formation, were induced by the exogenous NO donor S-nitrosoglutathione (GSNO) under the +P + Fe condition, but were inhibited by the NO-specific endogenous scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl- 3-oxide (cPTIO) under -P + Fe, +P-Fe and -P-Fe conditions. However, cluster roots induced by an exogenous supply of the NO donor did not secrete citrate, unlike those formed under -P or -Fe conditions.
Conclusions: NO plays an important role in the shared signalling pathway of the P- and Fe-deficiency-induced formation of cluster roots in white lupin.
Figures






Similar articles
-
Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin.New Phytol. 2010 Sep;187(4):1112-1123. doi: 10.1111/j.1469-8137.2010.03323.x. Epub 2010 Jun 14. New Phytol. 2010. PMID: 20553395
-
Physiological and transcriptomic data highlight common features between iron and phosphorus acquisition mechanisms in white lupin roots.Plant Sci. 2019 Aug;285:110-121. doi: 10.1016/j.plantsci.2019.04.026. Epub 2019 May 3. Plant Sci. 2019. PMID: 31203875
-
Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.).J Exp Bot. 2014 Jul;65(12):2995-3003. doi: 10.1093/jxb/eru135. Epub 2014 Apr 10. J Exp Bot. 2014. PMID: 24723402 Free PMC article.
-
Update on lupin cluster roots. Update on white lupin cluster root acclimation to phosphorus deficiency.Plant Physiol. 2011 Jul;156(3):1025-32. doi: 10.1104/pp.111.175174. Epub 2011 Apr 4. Plant Physiol. 2011. PMID: 21464472 Free PMC article. Review. No abstract available.
-
Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants.Int J Mol Sci. 2021 May 5;22(9):4904. doi: 10.3390/ijms22094904. Int J Mol Sci. 2021. PMID: 34063156 Free PMC article. Review.
Cited by
-
Nitrate: A Crucial Signal during Lateral Roots Development.Front Plant Sci. 2017 Apr 4;8:485. doi: 10.3389/fpls.2017.00485. eCollection 2017. Front Plant Sci. 2017. PMID: 28421105 Free PMC article. Review.
-
Nitric oxide as a key component in hormone-regulated processes.Plant Cell Rep. 2013 Jun;32(6):853-66. doi: 10.1007/s00299-013-1434-1. Epub 2013 Apr 13. Plant Cell Rep. 2013. PMID: 23584547 Review.
-
Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress.Environ Sci Pollut Res Int. 2017 Jan;24(3):2273-2285. doi: 10.1007/s11356-016-7947-8. Epub 2016 Nov 3. Environ Sci Pollut Res Int. 2017. PMID: 27812964 Review.
-
Multiple roles of nitric oxide in root development and nitrogen uptake.Plant Signal Behav. 2017 Jan 2;12(1):e1274480. doi: 10.1080/15592324.2016.1274480. Plant Signal Behav. 2017. PMID: 28027007 Free PMC article.
-
The white lupin trehalase gene LaTRE1 regulates cluster root formation and function under phosphorus deficiency.Plant Physiol. 2024 Dec 2;196(4):2184-2198. doi: 10.1093/plphys/kiae290. Plant Physiol. 2024. PMID: 38805210 Free PMC article.
References
-
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. - PubMed
-
- Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. Journal of Experimental Botany. 2006;57:581–588. - PubMed
-
- Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A. Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. Journal of Experimental Botany. 2008;59:155–163. - PubMed
-
- Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta. 2005;221:297–303. - PubMed

