Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana
- PMID: 22351490
- PMCID: PMC3535166
- DOI: 10.1007/s12192-012-0330-x
Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana
Abstract
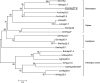
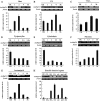
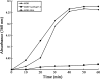
Small heat shock proteins (sHSPs) play an important role in the cellular defense of prokaryotic and eukaryotic organisms against a variety of internal and external stressors. In this study, a cDNA clone encoding a member of the α-crystallin/sHSP family, termed AccHsp27.6, was isolated from Apis cerana cerana. The full-length cDNA is 1,014 bp in length and contains a 708-bp open reading frame encoding a protein of 236 amino acids with a calculated molecular weight of 27.6 kDa and an isoelectric point of 7.53. Seven putative heat shock elements and three NF-κB binding sites were present in the 5'-flanking region, suggesting a possible function in immunity. A semi-quantitative RT-PCR analysis indicated that AccHsp27.6 was expressed in all tested tissues and at different developmental stages. Furthermore, expression of the AccHsp27.6 transcript was induced by exposure to heat shock, H(2)O(2), a number of different chemicals (including SO(2), formaldehyde, alcohol, acetone, chloroform, and the pesticides phoxime and acetamiprid), and the microbes Staphylococcus aureus and Micrococcus luteus. In contrast, the mRNA expression could be repressed by CO(2), the pesticides pyriproxyfen and cyhalothrin, and the microbes Bacillus subtilis and Pseudomonas aeruginosa. Notably, the recombinant AccHsp27.6 protein exhibited significant in vitro molecular chaperone activity and antimicrobial activity. Taken together, these results suggest that AccHsp27.6 might play an important role in the response to abiotic and biotic stresses and in immune reactions.
Figures








Similar articles
-
Two small heat shock protein genes in Apis cerana cerana: characterization, regulation, and developmental expression.Gene. 2014 Jul 25;545(2):205-14. doi: 10.1016/j.gene.2014.05.034. Epub 2014 May 15. Gene. 2014. PMID: 24835315
-
sHsp22.6, an intronless small heat shock protein gene, is involved in stress defence and development in Apis cerana cerana.Insect Biochem Mol Biol. 2014 Oct;53:1-12. doi: 10.1016/j.ibmb.2014.06.007. Epub 2014 Jul 5. Insect Biochem Mol Biol. 2014. PMID: 25008786
-
Identification and characterisation of a novel 1-Cys thioredoxin peroxidase gene (AccTpx5) from Apis cerana cerana.Comp Biochem Physiol B Biochem Mol Biol. 2014 Jun-Jul;172-173:39-48. doi: 10.1016/j.cbpb.2014.04.004. Epub 2014 Apr 16. Comp Biochem Physiol B Biochem Mol Biol. 2014. PMID: 24747012
-
Molecular cloning, expression and antioxidant characterisation of a typical thioredoxin gene (AccTrx2) in Apis cerana cerana.Gene. 2013 Sep 15;527(1):33-41. doi: 10.1016/j.gene.2013.05.062. Epub 2013 Jun 7. Gene. 2013. PMID: 23747404
-
Chaperone-like activity of alpha-crystallin and other small heat shock proteins.Curr Protein Pept Sci. 2001 Sep;2(3):205-25. doi: 10.2174/1389203013381107. Curr Protein Pept Sci. 2001. PMID: 12369933 Review.
Cited by
-
Characteristics and expression of genes encoding two small heat shock protein genes lacking introns from Chilo suppressalis.Cell Stress Chaperones. 2018 Jan;23(1):55-64. doi: 10.1007/s12192-017-0823-8. Epub 2017 Jul 7. Cell Stress Chaperones. 2018. PMID: 28687981 Free PMC article.
-
Identification and expression analysis of heat shock protein family genes of gall fly (Procecidochares utilis) under temperature stress.Cell Stress Chaperones. 2023 May;28(3):303-320. doi: 10.1007/s12192-023-01338-9. Epub 2023 Apr 18. Cell Stress Chaperones. 2023. PMID: 37071342 Free PMC article.
-
Impact of phosphorylation of heat shock protein 27 on the expression profile of periodontal ligament fibroblasts during mechanical strain.J Orofac Orthop. 2023 Apr;84(Suppl 2):143-153. doi: 10.1007/s00056-022-00391-w. Epub 2022 Apr 21. J Orofac Orthop. 2023. PMID: 35445818 Free PMC article.
-
Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy.Cells. 2019 Dec 24;9(1):60. doi: 10.3390/cells9010060. Cells. 2019. PMID: 31878360 Free PMC article. Review.
-
Characterization of small HSPs from Anemonia viridis reveals insights into molecular evolution of alpha crystallin genes among cnidarians.PLoS One. 2014 Sep 24;9(9):e105908. doi: 10.1371/journal.pone.0105908. eCollection 2014. PLoS One. 2014. PMID: 25251681 Free PMC article.
References
-
- Arrigo AP. Small stress proteins: chaperons that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

