Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs
- PMID: 22351492
- PMCID: PMC3314105
- DOI: 10.1007/s12350-012-9512-2
Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs
Abstract
Cardiotoxicity due to administration of cancer therapeutic agents such as anthracyclines and herceptin are well described. Established guidelines to screen for chemotherapy-related cardiotoxicity (CRC) are primarily based on serial assessment of left ventricular (LV) ejection fraction (EF). However, other parameters such as LV volume, diastolic function, and strain may also be useful in screening for cardiotoxicity. More recent advances in molecular imaging of apoptosis and tissue characterization by cardiac MRI are techniques which might allow early detection of patients at high risk for developing cardiotoxicity prior to a drop in EF. This comprehensive multi-modality review will discuss both the current established imaging techniques as well as the emerging technologies which may revolutionize the future of screening and evaluation for CRC.
Figures


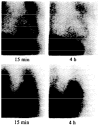
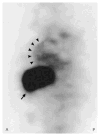
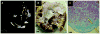

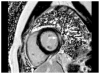
References
-
- Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiomyopathy and its prevention. Mol Genet Metab. 2000;71:436–44. - PubMed
-
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: The critical role of energetics. J Mol Cell Cardiol. 2006;41:389–405. - PubMed
-
- Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–7. - PubMed
-
- Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein polymorphisms are associated with doxorubicin-induced cardiomyopathy. Circulation. 2005;112:3754–62. - PubMed
-
- Minotti G, Saponiero L, Licata S, Menna P, Calafiore AM, Teodori G, et al. Paclitaxel and docetaxel enhance the metabolism of doxorubicin to toxic species in human myocardium. Clin Cancer Res. 2001;7:1511–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

