Contrarily to whey and high protein diets, dietary free leucine supplementation cannot reverse the lack of recovery of muscle mass after prolonged immobilization during ageing
- PMID: 22351629
- PMCID: PMC3573319
- DOI: 10.1113/jphysiol.2011.226266
Contrarily to whey and high protein diets, dietary free leucine supplementation cannot reverse the lack of recovery of muscle mass after prolonged immobilization during ageing
Abstract
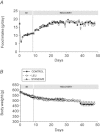
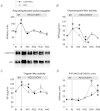
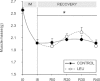
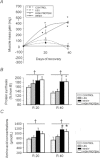
During ageing, immobilization periods increase and are partially responsible of sarcopaenia by inducing a muscle atrophy which is hardly recovered from. Immobilization-induced atrophy is due to an increase of muscle apoptotic and proteolytic processes and decreased protein synthesis. Moreover, previous data suggested that the lack of muscle mass recovery might be due to a defect in protein synthesis response during rehabilitation. This study was conducted to explore protein synthesis during reloading and leucine supplementation effect as a nutritional strategy for muscle recovery. Old rats (22–24 months old) were subjected to unilateral hindlimb casting for 8 days (I8) and allowed to recover for 10–40 days (R10–R40). They were fed a casein (±leucine) diet during the recovery. Immobilized gastrocnemius muscles atrophied by 20%, and did not recover even at R40. Amount of polyubiquitinated conjugates and chymotrypsin- and trypsin-like activities of the 26S proteasome increased. These changes paralleled an ‘anabolic resistance' of the protein synthesis at the postprandial state (decrease of protein synthesis, P-S6 and P-4E-BP1). During the recovery, proteasome activities remained elevated until R10 before complete normalization and protein synthesis was slightly increased. With free leucine supplementation during recovery, if proteasome activities were normalized earlier and protein synthesis was higher during the whole recovery, it nevertheless failed in muscle mass gain. This discrepancy could be due to a ‘desynchronization' between the leucine signal and the availability of amino acids coming from casein digestion. Thus, when supplemented with leucine-rich proteins (i.e. whey) and high protein diets, animals partially recovered the muscle mass loss.
Figures

Comment in
-
Leucine: a nutrient 'trigger' for muscle anabolism, but what more?J Physiol. 2012 May 1;590(9):2065-6. doi: 10.1113/jphysiol.2012.230631. J Physiol. 2012. PMID: 22548909 Free PMC article. No abstract available.
-
Contributions to the understanding of the anabolic properties of different dietary proteins.J Physiol. 2012 Jun 15;590(12):2839-40. doi: 10.1113/jphysiol.2012.233684. J Physiol. 2012. PMID: 22707594 Free PMC article. No abstract available.
References
-
- Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr. 1999;129:1102–1106. - PubMed
-
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. - PubMed
-
- Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr. 2001;131:1171–1176. - PubMed
-
- Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care. 2010;13:265–270. - PubMed
-
- Balage M, Averous J, Remond D, Bos C, Pujos-Guillot E, Papet I, Mosoni L, Combaret L, Dardevet D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2009;21:325–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

