Obesity and adipokines: effects on sympathetic overactivity
- PMID: 22351630
- PMCID: PMC3573303
- DOI: 10.1113/jphysiol.2011.221036
Obesity and adipokines: effects on sympathetic overactivity
Abstract
Excess body weight is a major risk factor for cardiovascular disease, increasing the risk of hypertension, hyperglycaemia and dyslipidaemia, recognized as the metabolic syndrome. Adipose tissue acts as an endocrine organ by producing various signalling cytokines called adipokines (including leptin, free fatty acids, tumour necrosis factor-α, interleukin-6, C-reactive protein, angiotensinogen and adiponectin). A chronic dysregulation of certain adipokines can have deleterious effects on insulin signalling. Chronic sympathetic overactivity is also known to be present in central obesity, and recent findings demonstrate the consequence of an elevated sympathetic outflow to organs such as the heart, kidneys and blood vessels. Chronic sympathetic nervous system overactivity can also contribute to a further decline of insulin sensitivity, creating a vicious cycle that may contribute to the development of the metabolic syndrome and hypertension. The cause of this overactivity is not clear, but may be driven by certain adipokines. The purpose of this review is to summarize how obesity, notably central or visceral as observed in the metabolic syndrome, leads to adipokine expression contributing to changes in insulin sensitivity and overactivity of the sympathetic nervous system.
Figures

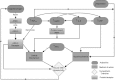
References
-
- Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–418. - PubMed
-
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. - PubMed
-
- Alvarez GE, Davy BM, Ballard TP, Beske SD, Davy KP. Weight loss increases cardiovagal baroreflex function in obese young and older men. Am J Physiol Endocrinol Metab. 2005;289:E665–669. - PubMed
-
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

