Are all spinal segments equal: intrinsic membrane properties of superficial dorsal horn neurons in the developing and mature mouse spinal cord
- PMID: 22351631
- PMCID: PMC3424761
- DOI: 10.1113/jphysiol.2012.227389
Are all spinal segments equal: intrinsic membrane properties of superficial dorsal horn neurons in the developing and mature mouse spinal cord
Abstract
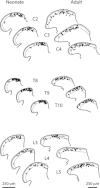
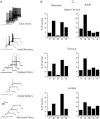
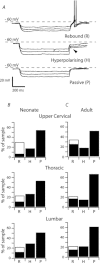
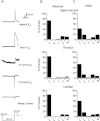
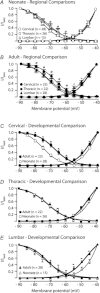
Neurons in the superficial dorsal horn (SDH; laminae I-II) of the spinal cord process nociceptive information from skin, muscle, joints and viscera. Most of what we know about the intrinsic properties of SDH neurons comes from studies in lumbar segments of the cord even though clinical evidence suggests nociceptive signals from viscera and head and neck tissues are processed differently. This ‘lumbar-centric' view of spinal pain processing mechanisms also applies to developing SDH neurons. Here we ask whether the intrinsic membrane properties of SDH neurons differ across spinal cord segments in both the developing and mature spinal cord. Whole cell recordings were made from SDH neurons in slices of upper cervical (C2-4), thoracic (T8-10) and lumbar (L3-5) segments in neonatal (P0-5) and adult (P24-45) mice. Neuronal input resistance (R(IN)), resting membrane potential, AP amplitude, half-width and AHP amplitude were similar across spinal cord regions in both neonates and adults (∼100 neurons for each region and age). In contrast, these intrinsic membrane properties differed dramatically between neonates and adults. Five types of AP discharge were observed during depolarizing current injection. In neonates, single spiking dominated (∼40%) and the proportions of each discharge category did not differ across spinal regions. In adults, initial bursting dominated in each spinal region, but was significantly more prevalent in rostral segments (49% of neurons in C2-4 vs. 29% in L3-5). During development the dominant AP discharge pattern changed from single spiking to initial bursting. The rapid A-type potassium current (I(Ar)) dominated in neonates and adults, but its prevalence decreased (∼80% vs. ∼50% of neurons) in all regions during development. I(Ar) steady state inactivation and activation also changed in upper cervical and lumbar regions during development. Together, our data show the intrinsic properties of SDH neurons are generally conserved in the three spinal cord regions examined in both neonate and adult mice. We propose the conserved intrinsic membrane properties of SDH neurons along the length of the spinal cord cannot explain the marked differences in pain experienced in the limbs, viscera, and head and neck.
Figures
Similar articles
-
Evidence for a critical period in the development of excitability and potassium currents in mouse lumbar superficial dorsal horn neurons.J Neurophysiol. 2009 Apr;101(4):1800-12. doi: 10.1152/jn.90755.2008. Epub 2009 Jan 28. J Neurophysiol. 2009. PMID: 19176612
-
Recording temperature affects the excitability of mouse superficial dorsal horn neurons, in vitro.J Neurophysiol. 2008 May;99(5):2048-59. doi: 10.1152/jn.01176.2007. Epub 2008 Feb 20. J Neurophysiol. 2008. PMID: 18287548
-
Neonatal tissue injury reduces the intrinsic excitability of adult mouse superficial dorsal horn neurons.Neuroscience. 2014 Jan 3;256:392-402. doi: 10.1016/j.neuroscience.2013.10.057. Epub 2013 Nov 1. Neuroscience. 2014. PMID: 24184978 Free PMC article.
-
Moving from an averaged to specific view of spinal cord pain processing circuits.J Neurophysiol. 2007 Sep;98(3):1057-63. doi: 10.1152/jn.00581.2007. Epub 2007 Jun 13. J Neurophysiol. 2007. PMID: 17567772 Review.
-
The synaptic connectivity that underlies the noxious transmission and modulation within the superficial dorsal horn of the spinal cord.Prog Neurobiol. 2010 May;91(1):38-54. doi: 10.1016/j.pneurobio.2010.01.005. Epub 2010 Jan 25. Prog Neurobiol. 2010. PMID: 20100541 Review.
Cited by
-
A Novel Anti-Inflammatory d-Peptide Inhibits Disease Phenotype Progression in an ALS Mouse Model.Molecules. 2021 Mar 13;26(6):1590. doi: 10.3390/molecules26061590. Molecules. 2021. PMID: 33805709 Free PMC article.
-
Functional subpopulations of V3 interneurons in the mature mouse spinal cord.J Neurosci. 2013 Nov 20;33(47):18553-65. doi: 10.1523/JNEUROSCI.2005-13.2013. J Neurosci. 2013. PMID: 24259577 Free PMC article.
-
Oral Treatment with RD2RD2 Impedes Development of Motoric Phenotype and Delays Symptom Onset in SOD1G93A Transgenic Mice.Int J Mol Sci. 2021 Jun 30;22(13):7066. doi: 10.3390/ijms22137066. Int J Mol Sci. 2021. PMID: 34209129 Free PMC article.
-
Contrasting alterations to synaptic and intrinsic properties in upper-cervical superficial dorsal horn neurons following acute neck muscle inflammation.Mol Pain. 2014 Apr 12;10:25. doi: 10.1186/1744-8069-10-25. Mol Pain. 2014. PMID: 24725960 Free PMC article.
-
Acute inhibition of acid sensing ion channel 1a after spinal cord injury selectively affects excitatory synaptic transmission, but not intrinsic membrane properties, in deep dorsal horn interneurons.PLoS One. 2023 Nov 8;18(11):e0289053. doi: 10.1371/journal.pone.0289053. eCollection 2023. PLoS One. 2023. PMID: 37939057 Free PMC article.
References
-
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. - PubMed
-
- Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58:283–307. - PubMed
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

