New advances in MR-compatible bioartificial liver
- PMID: 22351642
- PMCID: PMC4332620
- DOI: 10.1002/nbm.1633
New advances in MR-compatible bioartificial liver
Abstract
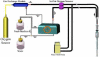
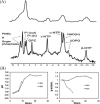
MR-compatible bioartificial liver (BAL) studies have been performed for 30 years and are reviewed. There are two types of study: (i) metabolism and drug studies using multinuclear MRS; primarily short-term (< 8 h) studies; (ii) the use of multinuclear MRS and MRI to noninvasively define the features and functions of BAL systems for long-term liver tissue engineering. In the latter, these systems often undergo not only modification of the perfusion system, but also the construction of MR radiofrequency probes around the bioreactor. We present novel MR-compatible BALs and the use of multinuclear MRS ((13)C, (19)F, (31)P) for the noninvasive monitoring of their growth, metabolism and viability, as well as (1)H MRI methods for the determination of flow profiles, diffusion, cell distribution, quality assurance and bioreactor integrity. Finally, a simple flexible coil design and circuit, and life support system, are described that can make almost any BAL MR-compatible.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures








Similar articles
-
Perfusion flow rate substantially contributes to the performance of the HepaRG-AMC-bioartificial liver.Biotechnol Bioeng. 2012 Dec;109(12):3182-8. doi: 10.1002/bit.24586. Epub 2012 Jul 4. Biotechnol Bioeng. 2012. PMID: 22729831
-
[Control and realization of a novel bioartificial liver support system].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008 Apr;25(2):445-9. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008. PMID: 18610639 Chinese.
-
Sustaining a bioartificial liver under hypothermic conditions.Tissue Eng. 2005 Mar-Apr;11(3-4):427-37. doi: 10.1089/ten.2005.11.427. Tissue Eng. 2005. PMID: 15869421
-
Bioartificial liver systems: current status and future perspective.J Biosci Bioeng. 2005 Apr;99(4):311-9. doi: 10.1263/jbb.99.311. J Biosci Bioeng. 2005. PMID: 16233796 Review.
-
Integration of technologies for hepatic tissue engineering.Adv Biochem Eng Biotechnol. 2007;103:309-29. doi: 10.1007/10_029. Adv Biochem Eng Biotechnol. 2007. PMID: 17195468 Review.
Cited by
-
In vitro models for liver toxicity testing.Toxicol Res (Camb). 2013 Jan 1;2(1):23-39. doi: 10.1039/C2TX20051A. Epub 2012 Nov 23. Toxicol Res (Camb). 2013. PMID: 23495363 Free PMC article.
-
Effect of oxygen concentration on viability and metabolism in a fluidized-bed bioartificial liver using ³¹P and ¹³C NMR spectroscopy.Tissue Eng Part C Methods. 2013 Feb;19(2):93-100. doi: 10.1089/ten.TEC.2011.0629. Epub 2012 Sep 28. Tissue Eng Part C Methods. 2013. PMID: 22835003 Free PMC article.
References
-
- Dale BE, Gillies RJ. Nuclear magnetic resonance spectroscopy of dense cell populations for metabolic studies and bioreactor engineering: a synergistic partnership. Biotechnology. 1991;17:107–118. - PubMed
-
- Ruiz-Cabello J, Cohen JS. NMR and the study of pathological state in cells and tissues. Int. Rev. Cytol. 1993;145:1–63. - PubMed
-
- Kaplan O, Cohen JS. Metabolism of breast cancer cells as revealed by non-invasive magnetic resonance spectroscopy studies. Breast Cancer Res. Treat. 1994;31(2–3):285–299. - PubMed
-
- Szwergold BS. NMR spectroscopy of cells. Annu. Rev. Physiol. 1992;54:775–798. - PubMed
-
- Zupke C, Foy B. Nuclear magnetic resonance analysis of cell metabolism. Curr. Opin. Biotechnol. 1995;6(2):192–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

