New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis
- PMID: 22351713
- PMCID: PMC3586733
- DOI: 10.7326/0003-4819-156-4-201202210-00005
New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis
Abstract
Background: Chronic hepatitis C virus is difficult to treat and affects approximately 3 million Americans. Protease inhibitors increase the effectiveness of standard therapy, but they are costly. A genetic assay may identify patients most likely to benefit from this treatment advance.
Objective: To assess the cost-effectiveness of new protease inhibitors and an interleukin (IL)-28B genotyping assay for treating chronic hepatitis C virus.
Design: Decision-analytic Markov model.
Data sources: Published literature and expert opinion.
Target population: Treatment-naive patients with chronic, genotype 1 hepatitis C virus monoinfection.
Time horizon: Lifetime.
Perspective: Societal.
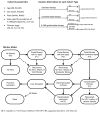
Intervention: Strategies are defined by the use of IL-28B genotyping and type of treatment (standard therapy [pegylated interferon with ribavirin]; triple therapy [standard therapy and a protease inhibitor]). Interleukin-28B-guided triple therapy stratifies patients with CC genotypes to standard therapy and those with non-CC types to triple therapy.
Outcome measures: Discounted costs (in 2010 U.S. dollars) and quality-adjusted life-years (QALYs); incremental cost-effectiveness ratios.
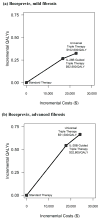
Results of base-case analysis: For patients with mild and advanced fibrosis, universal triple therapy reduced the lifetime risk for hepatocellular carcinoma by 38% and 28%, respectively, and increased quality-adjusted life expectancy by 3% and 8%, respectively, compared with standard therapy. Gains from IL-28B-guided triple therapy were smaller. If the protease inhibitor costs $1100 per week, universal triple therapy costs $102,600 per QALY (mild fibrosis) or $51,500 per QALY (advanced fibrosis) compared with IL-28B-guided triple therapy and $70,100 per QALY (mild fibrosis) and $36,300 per QALY (advanced fibrosis) compared with standard therapy.
Results of sensitivity analysis: Results were sensitive to the cost of protease inhibitors and treatment adherence rates.
Limitation: Data on the long-term comparative effectiveness of the new protease inhibitors are lacking.
Conclusion: Both universal triple therapy and IL-28B-guided triple therapy are cost-effective when the least-expensive protease inhibitor are used for patients with advanced fibrosis.
Primary funding source: Stanford University.
Conflict of interest statement
Figures

Comment in
-
Hepatitis C: the end of the beginning and possibly the beginning of the end.Ann Intern Med. 2012 Feb 21;156(4):317-8. doi: 10.7326/0003-4819-156-4-201202210-00014. Ann Intern Med. 2012. PMID: 22351718 Free PMC article. No abstract available.
References
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144(10):705–14. - PubMed
-
- Nainan OV, Alter MJ, Kruszon-Moran D, Gao FX, Xia G, McQuillan G, et al. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology. 2006;131(2):478–84. - PubMed
-
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection. New England Journal of Medicine. 2009;361(10):580. 1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
