Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma
- PMID: 22351749
- PMCID: PMC3309856
- DOI: 10.1093/neuonc/nos005
Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma
Abstract
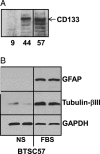
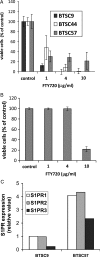
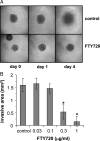
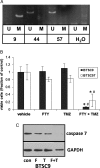
FTY720 is a sphingosine analogue that down regulates expression of sphingosine-1-phosphate receptors and causes apoptosis of multiple tumor cell types, including glioma cells. This study examined the effect of FTY720 on brain tumor stem cells (BTSCs) derived from human glioblastoma (GBM) tissue. FTY720 treatment of BTSCs led to rapid inactivation of ERK MAP kinase, leading to upregulation of the BH3-only protein Bim and apoptosis. In combination with temozolomide (TMZ), the current standard chemotherapeutic agent for GBM, FTY720 synergistically induced BTSC apoptosis. FTY720 also slowed growth of intracranial xenograft tumors in nude mice and augmented the therapeutic effect of TMZ, leading to enhanced survival. Furthermore, the combination of FTY720 and TMZ decreased the invasiveness of BTSCs in mouse brains. FTY720 is known to cross the blood-brain barrier and recently received Food and Drug Administration approval for treatment of relapsing multiple sclerosis. Thus, FTY720 is an excellent potential therapeutic agent for treatment of GBM.
Figures

Similar articles
-
FTY720 induces autophagy-related apoptosis and necroptosis in human glioblastoma cells.Toxicol Lett. 2015 Jul 2;236(1):43-59. doi: 10.1016/j.toxlet.2015.04.015. Epub 2015 May 1. Toxicol Lett. 2015. PMID: 25939952
-
FTY720 induces apoptosis of chronic myelogenous leukemia cells via dual activation of BIM and BID and overcomes various types of resistance to tyrosine kinase inhibitors.Apoptosis. 2013 Nov;18(11):1437-1446. doi: 10.1007/s10495-013-0882-y. Apoptosis. 2013. PMID: 23851982
-
Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats.Stroke. 2010 Feb;41(2):368-74. doi: 10.1161/STROKEAHA.109.568899. Epub 2009 Nov 25. Stroke. 2010. PMID: 19940275 Free PMC article.
-
Phosphorylation of the immunomodulator FTY720 inhibits programmed cell death of fibroblasts via the S1P3 receptor subtype and Bcl-2 activation.Cell Physiol Biochem. 2010;26(1):67-78. doi: 10.1159/000315107. Epub 2010 May 18. Cell Physiol Biochem. 2010. PMID: 20502006 Review.
-
Ascomycete derivative to MS therapeutic: S1P receptor modulator FTY720.Prog Drug Res. 2008;66:361, 363-81. doi: 10.1007/978-3-7643-8595-8_8. Prog Drug Res. 2008. PMID: 18416311 Review.
Cited by
-
A genome-wide screen for FTY720-sensitive mutants reveals genes required for ROS homeostasis.Microb Cell. 2017 Nov 27;4(12):390-401. doi: 10.15698/mic2017.12.601. Microb Cell. 2017. PMID: 29234668 Free PMC article.
-
Targeting survival pathways in chronic myeloid leukaemia stem cells.Br J Pharmacol. 2013 Aug;169(8):1693-707. doi: 10.1111/bph.12183. Br J Pharmacol. 2013. PMID: 23517124 Free PMC article. Review.
-
FTY720 inhibits tumor growth and enhances the tumor-suppressive effect of topotecan in neuroblastoma by interfering with the sphingolipid signaling pathway.Pediatr Blood Cancer. 2013 Sep;60(9):1418-23. doi: 10.1002/pbc.24564. Epub 2013 May 23. Pediatr Blood Cancer. 2013. PMID: 23704073 Free PMC article.
-
Sphingosine 1-Phosphate Receptors and Metabolic Enzymes as Druggable Targets for Brain Diseases.Front Pharmacol. 2019 Jul 23;10:807. doi: 10.3389/fphar.2019.00807. eCollection 2019. Front Pharmacol. 2019. PMID: 31427962 Free PMC article. Review.
-
FTY720 Inhibits Expansion of Breast Cancer Stem Cells via PP2A Activation.Int J Mol Sci. 2021 Jul 6;22(14):7259. doi: 10.3390/ijms22147259. Int J Mol Sci. 2021. PMID: 34298877 Free PMC article.
References
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi:10.1038/nrm1103. - DOI - PubMed
-
- Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. - PubMed
-
- Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme. Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi:10.1097/01.jnen.0000175329.59092.2c. - DOI - PubMed
-
- Van Brocklyn JR, Letterle CA, Snyder PJ, Prior TW. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: Role of ERK MAP kinase and phosphatidylinositol 3-kinase β. Cancer Lett. 2002;181:195–204. doi:10.1016/S0304-3835(02)00050-2. - DOI - PubMed
-
- Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi:10.1016/S0304-3835(03)00334-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

