Phosphatidylinositol 5-phosphatase oculocerebrorenal syndrome of Lowe protein (OCRL) controls actin dynamics during early steps of Listeria monocytogenes infection
- PMID: 22351770
- PMCID: PMC3339978
- DOI: 10.1074/jbc.M111.315788
Phosphatidylinositol 5-phosphatase oculocerebrorenal syndrome of Lowe protein (OCRL) controls actin dynamics during early steps of Listeria monocytogenes infection
Abstract
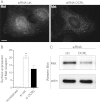
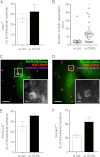
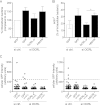
Listeria monocytogenes is a bacterial pathogen that induces its own entry into a broad range of mammalian cells through interaction of the bacterial surface protein InlB with the cellular receptor Met, promoting an actin polymerization/depolymerization process that leads to pathogen engulfment. Phosphatidylinositol bisphosphate (PI[4,5]P(2)) and trisphosphate (PI[3,4,5]P(3)) are two major phosphoinositide species that function as molecular scaffolds, recruiting cellular effectors that regulate actin dynamics during L. monocytogenes infection. Because the phosphatidylinositol 5'-phosphatase OCRL dephosphorylates PI(4,5)P(2) and to a lesser extent PI(3,4,5)P(3), we investigated whether this phosphatase modulates cell invasion by L. monocytogenes. Inactivation of OCRL by small interfering RNA (siRNA) leads to an increase in the internalization levels of L. monocytogenes in HeLa cells. Interestingly, OCRL depletion does not increase but rather decreases the surface expression of the receptor Met, suggesting that OCRL controls bacterial internalization by modulating signaling cascades downstream of Met. Immuno-fluorescence microscopy reveals that endogenous and overexpressed OCRL are present at L. monocytogenes invasion foci; live-cell imaging additionally shows that actin depolymerization coincides with EGFP-OCRL-a accumulation around invading bacteria. Together, these observations suggest that OCRL promotes actin depolymerization during L. monocytogenes infection; in agreement with this hypothesis, OCRL depletion leads to an increase in actin, PI(4,5)P(2), and PI(3,4,5)P(3) levels at bacterial internalization foci. Furthermore, in cells knocked down for OCRL, transfection of enzymatically active EGFP-OCRL-a (but not of a phosphatase-dead enzyme) decreases the levels of intracellular L. monocytogenes and of actin associated with invading bacteria. These results demonstrate that through its phosphatase activity, OCRL restricts L. monocytogenes invasion by modulating actin dynamics at bacterial internalization sites.
Figures



References
-
- Stavru F., Archambaud C., Cossart P. (2011) Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol. Rev. 240, 160–184 - PubMed
-
- Mengaud J., Ohayon H., Gounon P., Mege R.-M., Cossart P. (1996) E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923–932 - PubMed
-
- Shen Y., Naujokas M., Park M., Ireton K. (2000) InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103, 501–510 - PubMed
-
- Pizarro-Cerdá J., Cossart P. (2009) Listeria monocytogenes membrane trafficking and lifestyle: the exception or the rule? Annu. Rev. Cell Dev. Biol. 25, 649–670 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

