Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis
- PMID: 22351773
- PMCID: PMC3320944
- DOI: 10.1074/jbc.M111.336834
Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis
Abstract
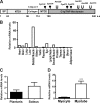
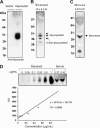
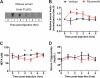
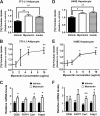
Skeletal muscle plays important roles in whole-body glucose and fatty acid metabolism. However, muscle also secretes cytokines and growth factors (collectively termed myokines) that can potentially act in an autocrine, a paracrine, and/or an endocrine manner to modulate metabolic, inflammatory, and other processes. Here, we report the identification and characterization of myonectin, a novel myokine belonging to the C1q/TNF-related protein (CTRP) family. Myonectin transcript was highly induced in differentiated myotubes and predominantly expressed by skeletal muscle. Circulating levels of myonectin were tightly regulated by the metabolic state; fasting suppressed, but refeeding dramatically increased, its mRNA and serum levels. Although mRNA and circulating levels of myonectin were reduced in a diet-induced obese state, voluntary exercise increased its expression and circulating levels. Accordingly, myonectin transcript was up-regulated by compounds (forskolin, epinephrine, ionomycin) that raise cellular cAMP or calcium levels. In vitro, secreted myonectin forms disulfide-linked oligomers, and when co-expressed, forms heteromeric complexes with other members of the C1q/TNF-related protein family. In mice, recombinant myonectin administration reduced circulating levels of free fatty acids without altering adipose tissue lipolysis. Consistent with this, myonectin promoted fatty acid uptake in cultured adipocytes and hepatocytes, in part by up-regulating the expression of genes (CD36, FATP1, Fabp1, and Fabp4) that promote lipid uptake. Collectively, these results suggest that myonectin links skeletal muscle to lipid homeostasis in liver and adipose tissue in response to alterations in energy state, revealing a novel myonectin-mediated metabolic circuit.
Figures






References
-
- Wasserman D. H., Ayala J. E. (2005) Interaction of physiological mechanisms in control of muscle glucose uptake. Clin. Exp. Pharmacol. Physiol. 32, 319–323 - PubMed
-
- Rasmussen B. B., Wolfe R. R. (1999) Regulation of fatty acid oxidation in skeletal muscle. Annu. Rev. Nutr. 19, 463–484 - PubMed
-
- Petersen K. F., Shulman G. I. (2002) Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am. J. Cardiol. 90, 11G–18G - PubMed
-
- Pedersen B. K. (2009) Edward F. Adolph distinguished lecture: muscle as an endocrine organ: IL-6 and other myokines. J. Appl. Physiol. 107, 1006–1014 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

