Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma
- PMID: 22351932
- PMCID: PMC3302221
- DOI: 10.1084/jem.20111923
Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma
Abstract
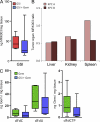
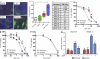
Pancreatic ductal adenocarcinoma (PDA) is a highly lethal disease that is refractory to medical intervention. Notch pathway antagonism has been shown to prevent pancreatic preneoplasia progression in mouse models, but potential benefits in the setting of an established PDA tumor have not been established. We demonstrate that the gamma secretase inhibitor MRK003 effectively inhibits intratumoral Notch signaling in the KPC mouse model of advanced PDA. Although MRK003 monotherapy fails to extend the lifespan of KPC mice, the combination of MRK003 with the chemotherapeutic gemcitabine prolongs survival. Combination treatment kills tumor endothelial cells and synergistically promotes widespread hypoxic necrosis. These results indicate that the paucivascular nature of PDA can be exploited as a therapeutic vulnerability, and the dual targeting of the tumor endothelium and neoplastic cells by gamma secretase inhibition constitutes a rationale for clinical translation.
Figures



References
-
- Bapiro T.E., Richards F.M., Goldgraben M.A., Olive K.P., Madhu B., Frese K.K., Cook N., Jacobetz M.A., Smith D.M., Tuveson D.A., et al. 2011. A novel method for quantification of gemcitabine and its metabolites 2′,2′-difluorodeoxyuridine and gemcitabine triphosphate in tumour tissue by LC-MS/MS: comparison with (19)F NMR spectroscopy. Cancer Chemother. Pharmacol. 68:1243–1253 10.1007/s00280-011-1613-0 - DOI - PMC - PubMed
-
- Burris H.A., III, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. 1997. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15:2403–2413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

