Bone marrow-derived IL-13Rα1-positive thymic progenitors are restricted to the myeloid lineage
- PMID: 22351937
- PMCID: PMC3311706
- DOI: 10.4049/jimmunol.1103316
Bone marrow-derived IL-13Rα1-positive thymic progenitors are restricted to the myeloid lineage
Abstract
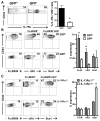
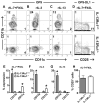
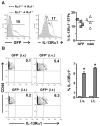
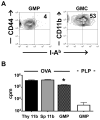
The earliest thymic progenitors (ETPs) were recently shown to give rise to both lymphoid and myeloid cells. Whereas the majority of ETPs are derived from IL-7Rα-positive cells and give rise exclusively to T cells, the origin of the myeloid cells remains undefined. In this study, we show both in vitro and in vivo that IL-13Rα1(+) ETPs yield myeloid cells with no potential for maturation into T cells, whereas IL-13Rα1(-) ETPs lack myeloid potential. Moreover, transfer of lineage-negative IL-13Rα1(+) bone marrow stem cells into IL-13Rα1-deficient mice reconstituted thymic IL-13Rα1(+) myeloid ETPs. Myeloid cells or macrophages in the thymus are regarded as phagocytic cells whose function is to clear apoptotic debris generated during T cell development. However, the myeloid cells derived from IL-13Rα1(+) ETPs were found to perform Ag-presenting functions. Thus, IL-13Rα1 defines a new class of myeloid restricted ETPs yielding APCs that could contribute to development of T cells and the control of immunity and autoimmunity.
Figures



Similar articles
-
A distinct dendritic cell population arises in the thymus of IL-13Rα1-sufficient but not IL-13Rα1-deficient mice.Cell Immunol. 2018 Sep;331:130-136. doi: 10.1016/j.cellimm.2018.06.006. Epub 2018 Jun 18. Cell Immunol. 2018. PMID: 29929727 Free PMC article.
-
IL-4/IL-13 Signaling Inhibits the Potential of Early Thymic Progenitors To Commit to the T Cell Lineage.J Immunol. 2017 Oct 15;199(8):2767-2776. doi: 10.4049/jimmunol.1700498. Epub 2017 Sep 11. J Immunol. 2017. PMID: 28893952 Free PMC article.
-
Interleukin-7 in the transition of bone marrow progenitors to the thymus.Immunol Cell Biol. 2017 Nov;95(10):916-924. doi: 10.1038/icb.2017.68. Epub 2017 Aug 16. Immunol Cell Biol. 2017. PMID: 28811625
-
Untangling the T branch of the hematopoiesis tree.Curr Opin Immunol. 2009 Apr;21(2):121-6. doi: 10.1016/j.coi.2009.01.012. Epub 2009 Mar 5. Curr Opin Immunol. 2009. PMID: 19269149 Free PMC article. Review.
-
Thymus-bound: the many features of T cell progenitors.Front Biosci (Schol Ed). 2011 Jun 1;3(3):961-9. doi: 10.2741/200. Front Biosci (Schol Ed). 2011. PMID: 21622245 Review.
Cited by
-
In Silico Integration of Transcriptome and Interactome Predicts an ETP-ALL-Specific Transcriptional Footprint that Decodes its Developmental Propensity.Front Cell Dev Biol. 2022 May 13;10:899752. doi: 10.3389/fcell.2022.899752. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646901 Free PMC article.
-
A distinct dendritic cell population arises in the thymus of IL-13Rα1-sufficient but not IL-13Rα1-deficient mice.Cell Immunol. 2018 Sep;331:130-136. doi: 10.1016/j.cellimm.2018.06.006. Epub 2018 Jun 18. Cell Immunol. 2018. PMID: 29929727 Free PMC article.
-
Interleukin-13 Receptor α1-Mediated Signaling Regulates Age-Associated/Autoimmune B Cell Expansion and Lupus Pathogenesis.Arthritis Rheumatol. 2022 Sep;74(9):1544-1555. doi: 10.1002/art.42146. Epub 2022 Aug 22. Arthritis Rheumatol. 2022. PMID: 35438841 Free PMC article.
-
Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses.Nat Immunol. 2016 Jan;17(1):57-64. doi: 10.1038/ni.3294. Epub 2015 Nov 2. Nat Immunol. 2016. PMID: 26523868 Free PMC article.
-
Neonatal Basophils Stifle the Function of Early-Life Dendritic Cells To Curtail Th1 Immunity in Newborn Mice.J Immunol. 2015 Jul 15;195(2):507-18. doi: 10.4049/jimmunol.1500027. Epub 2015 Jun 1. J Immunol. 2015. PMID: 26034171 Free PMC article.
References
-
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. - PubMed
-
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. - PubMed
-
- Godfrey DI, Zlotnik A, Suda T. Phenotypic and functional characterization of c-kit expression during intrathymic T cell development. J Immunol. 1992;149:2281–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

