Influence of acute hyperglycemia on otoacoustic emissions and the medial olivocochlear reflex
- PMID: 22352503
- PMCID: PMC3292605
- DOI: 10.1121/1.3676609
Influence of acute hyperglycemia on otoacoustic emissions and the medial olivocochlear reflex
Abstract
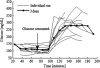
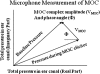
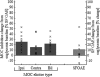
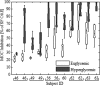
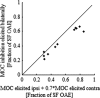
Stimulus-frequency (SF) otoacoustic emission (OAE) amplitude and the amplitude of medial olivocochlear (MOC) inhibition of SF OAEs for ipsilateral, contralateral and bilateral MOC reflex elicitors were recorded in six subjects with type 2 diabetes during a glucose tolerance test (GTT). Five of the six subjects were tested twice for a total of 11 trials and three subjects were tested in a control experiment. During the GTT experiment, the subjects' blood glucose was elevated from a euglycemic level below 150 mg/dL to a hyperglycemic level above 160 mg/dL following the consumption of a bolus of 80 g of sugar. A subset of three subjects were tested in a control experiment during which SF OAE and MOC reflex measurements were made while blood sugar levels remained constant within the euglycemic region. Mean SF OAE amplitudes were elevated following glucose consumption. A statistically significant increase in MOC inhibition amplitude was observed during elevated sugar levels for the 11 GTT trials. Maximum inhibition occurred about an hour after glucose consumption when blood glucose levels peaked. Results indicate that acute hyperglycemia influences efferent control of the cochlea in people with type 2 diabetes.
Figures


References
-
- Berlin, C. (2002). Hair Cell Micro-mechanics and Otoacoustic Emissions (Delmar Learning, Thomason Learning, Clifton Park, NY: ), pp. 1–155.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

