Neuromuscular control of fundamental frequency and glottal posture at phonation onset
- PMID: 22352513
- PMCID: PMC3292611
- DOI: 10.1121/1.3672686
Neuromuscular control of fundamental frequency and glottal posture at phonation onset
Abstract
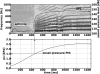
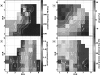
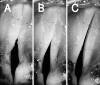
The laryngeal neuromuscular mechanisms for modulating glottal posture and fundamental frequency are of interest in understanding normal laryngeal physiology and treating vocal pathology. The intrinsic laryngeal muscles in an in vivo canine model were electrically activated in a graded fashion to investigate their effects on onset frequency, phonation onset pressure, vocal fold strain, and glottal distance at the vocal processes. Muscle activation plots for these laryngeal parameters were evaluated for the interaction of following pairs of muscle activation conditions: (1) cricothyroid (CT) versus all laryngeal adductors (TA/LCA/IA), (2) CT versus LCA/IA, (3) CT versus thyroarytenoid (TA) and, (4) TA versus LCA/IA (LCA: lateral cricoarytenoid muscle, IA: interarytenoid). Increases in onset frequency and strain were primarily affected by CT activation. Onset pressure correlated with activation of all adductors in activation condition 1, but primarily with CT activation in conditions 2 and 3. TA and CT were antagonistic for strain. LCA/IA activation primarily closed the cartilaginous glottis while TA activation closed the mid-membranous glottis.
© 2012 Acoustical Society of America
Figures







References
-
- Choi, H. S., Berke, G. S., Ye, M., and Kreiman, J. (1993a). “Function of the thyroarytenoid muscle in a canine laryngeal model,” Ann. Otol. Rhinol. Laryngol. 102, 769–776. - PubMed
-
- Choi, H. S., Berke, G. S., Ye, M., and Kreiman J. (1993b). “Function of the posterior cricoarytenoid muscle in phonation: In vivo laryngeal model,” Otolaryngol. Head Neck Surg. 109(6), 1043–1051. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

