Investigating photoexcitation-induced mitochondrial damage by chemotherapeutic corroles using multimode optical imaging
- PMID: 22352647
- PMCID: PMC3380813
- DOI: 10.1117/1.JBO.17.1.015003
Investigating photoexcitation-induced mitochondrial damage by chemotherapeutic corroles using multimode optical imaging
Abstract
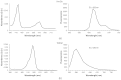
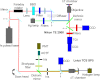
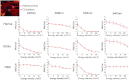
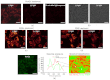
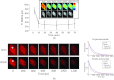
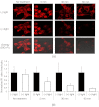
We recently reported that a targeted, brightly fluorescent gallium corrole (HerGa) is highly effective for breast tumor detection and treatment. Unlike structurally similar porphryins, HerGa exhibits tumor-targeted toxicity without the need for photoexcitation. We have now examined whether photoexcitation further modulates HerGa toxicity, using multimode optical imaging of live cells, including two-photon excited fluorescence, differential interference contrast (DIC), spectral, and lifetime imaging. Using two-photon excited fluorescence imaging, we observed that light at specific wavelengths augments the HerGa-mediated mitochondrial membrane potential disruption of breast cancer cells in situ. In addition, DIC, spectral, and fluorescence lifetime imaging enabled us to both validate cell damage by HerGa photoexcitation and investigate HerGa internalization, thus allowing optimization of light dose and timing. Our demonstration of HerGa phototoxicity opens the way for development of new methods of cancer intervention using tumor-targeted corroles.
Figures

References
-
- Agadjanian H., et al. , “Corrole Conjugates: A Unique Approach to Tumor Targeting,” in The American Association for Cancer Research, San Diego, CA, pp. 2328 (2008).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

