The electrophile responsive proteome: integrating proteomics and lipidomics with cellular function
- PMID: 22352679
- PMCID: PMC3448939
- DOI: 10.1089/ars.2012.4523
The electrophile responsive proteome: integrating proteomics and lipidomics with cellular function
Abstract
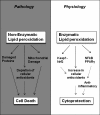
Significance: The process of lipid peroxidation is emerging as an important mechanism that mediates the post-translational modification of proteins. Through advanced analytical techniques, lipidomics is now emerging as a critical factor in our understanding of the pathology of a broad range of diseases.
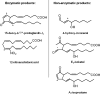
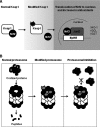
Recent advances: During enzymatic or nonenzymatic lipid peroxidation, the simple structure of an unsaturated fatty acid is converted to an oxylipidome, many members of which are electrophilic and form the reactive lipid species (RLS). This aspect of lipid biology is particularly important, as it directly connects lipidomics with proteomics through the post-translational modification of a sub-proteome in the cell. This arises, because the electrophilic members of the oxylipidome react with proteins at nucleophilic amino-acid residues and so change their structure and function to form electrophile-responsive proteomes (ERP).
Critical issues: Biological systems have relatively few but well-defined and mechanistically distinct pro-oxidant pathways generating RLS. Defining the ERPs and the mechanisms underlying their formation and action has been a major focus for the field of lipidomics and redox signaling.
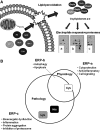
Future directions: We propose that a unique oxylipidome can be defined for specific oxidants and will predict the biological responses through the reaction with proteins to form a specific ERP. In this review, we will describe the ERPs that modulate antioxidant and anti-inflammatory protective pathways, including the activation of Keap1/Nrf2 and the promotion of cell death through interactions with mitochondria.
Figures

References
-
- Amer J. Ghoti H. Rachmilewitz E. Koren A. Levin C. Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132:108–113. - PubMed
-
- Basu S. Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7:221–235. - PubMed
-
- Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

