Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences
- PMID: 22352802
- PMCID: PMC3409651
- DOI: 10.1021/jf201987k
Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences
Abstract
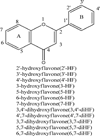
Sulfation and glucuronidation are the principal metabolic pathways of flavonoids, and extensive phase II metabolism is the main reason for their poor bioavailabilities. The purpose of this study was to compare the similarities and differences in the positional preference of glucuronidation versus sulfation in the mouse liver S9 fraction. The conjugating rates of seven monohydroxyflavones (HFs) (i.e., 2'-, 3'-, 4'-, 3-, 5-, 6-, and 7-HF), and five dihydroxyflavones (diHFs) (i.e., 6,7-, 4',7-, 3,7-, 5,7-, and 3,4'-diHF) were determined in three separate enzymatic reaction systems: (A) sulfation only, (B) glucuronidation only, or (C) simultaneous sulfation and glucuronidation (i.e., Sult-Ugt coreaction). In general, glucuronidation rates were much faster than sulfation rates. Among the HFs, 7-HF was the best substrate for both conjugation reactions, whereas 3-HF was rapidly glucuronidated but was not sulfated. As a result, the rank order of sulfation was very different from that of glucuronidation. Among the diHFs, regiospecific glucuronidation was limited to 7-OH and 3-OH positions, whereas regiospecific sulfation was limited to 7-OH and 4'-OH positions. Other positions (i.e., 6-OH and 5-OH) in diHFs were not conjugated. The positional preferences were essentially maintained in a Sult-Ugt coreaction system, although sulfation was surprisingly enhanced. Lastly, sulfation and glucuronidation displayed different regiospecific- and substrate-dependent characteristics. In conclusion, glucuronidation and sulfation shared the same preference for 7-OH position (of flavonoids) but displayed unique preference in other positions in that glucuronidation preferred the 3-OH position whereas sulfation preferred the 4'-OH position.
Figures






References
-
- Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120(3):451–458. - PubMed
-
- Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56(15):6185–6205. - PubMed
-
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90(2–3):157–177. - PubMed
-
- Moon JH, Nakata R, Oshima S, Inakuma T, Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R461–R467. - PubMed
-
- de Vries JH, Hollman PC, Meyboom S, Buysman MN, Zock PL, van Staveren WA, Katan MB. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am J Clin Nutr. 1998;68(1):60–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

