Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis
- PMID: 22352884
- PMCID: PMC3323110
- DOI: 10.1021/bi201628j
Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis
Abstract
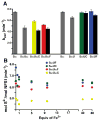
Human frataxin (FXN) has been intensively studied since the discovery that the FXN gene is associated with the neurodegenerative disease Friedreich's ataxia. Human FXN is a component of the NFS1-ISD11-ISCU2-FXN (SDUF) core Fe-S assembly complex and activates the cysteine desulfurase and Fe-S cluster biosynthesis reactions. In contrast, the Escherichia coli FXN homologue CyaY inhibits Fe-S cluster biosynthesis. To resolve this discrepancy, enzyme kinetic experiments were performed for the human and E. coli systems in which analogous cysteine desulfurase, Fe-S assembly scaffold, and frataxin components were interchanged. Surprisingly, our results reveal that activation or inhibition by the frataxin homologue is determined by which cysteine desulfurase is present and not by the identity of the frataxin homologue. These data are consistent with a model in which the frataxin-less Fe-S assembly complex exists as a mixture of functional and nonfunctional states, which are stabilized by binding of frataxin homologues. Intriguingly, this appears to be an unusual example in which modifications to an enzyme during evolution inverts or reverses the mode of control imparted by a regulatory molecule.
Figures


References
-
- Johnson DC, Dean DR, Smith AD, Johnson M. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. - PubMed
-
- Brzóska K, Meczyńska S, Kruszewski M. Iron-sulfur cluster proteins: electron transfer and beyond. Acta Biochim Pol. 2006;53:685–691. - PubMed
-
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. - PubMed
-
- Meyer J. Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J Biol Inorg Chem. 2008;13:157–170. - PubMed
-
- Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

