Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex
- PMID: 22352953
- PMCID: PMC5749646
- DOI: 10.1089/neu.2011.2207
Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex
Abstract
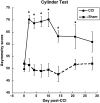
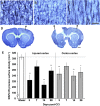
Compensatory neural plasticity occurs in both hemispheres following unilateral cortical damage incurred by seizures, stroke, and focal lesions. Plasticity is thought to play a role in recovery of function, and is important for the utility of rehabilitation strategies. Such effects have not been well described in models of traumatic brain injury (TBI). We examined changes in immunoreactivity for neural structural and plasticity-relevant proteins in the area surrounding a controlled cortical impact (CCI) to the forelimb sensorimotor cortex (FL-SMC), and in the contralateral homotopic cortex over time (3-28 days). CCI resulted in considerable motor deficits in the forelimb contralateral to injury, and increased reliance on the ipsilateral forelimb. The density of dendritic processes, visualized with immunostaining for microtubule-associated protein-2 (MAP-2), were bilaterally decreased at all time points. Synaptophysin (SYN) immunoreactivity increased transiently in the injured hemisphere, but this reflected an atypical labeling pattern, and it was unchanged in the contralateral hemisphere compared to uninjured controls. The lack of compensatory neuronal structural plasticity in the contralateral homotopic cortex, despite behavioral asymmetries, is in contrast to previous findings in stroke models. In the cortex surrounding the injury (but not the contralateral cortex), decreases in dendrites were accompanied by neurodegeneration, as indicated by Fluoro-Jade B (FJB) staining, and increased expression of the growth-inhibitory protein Nogo-A. These studies indicate that, following unilateral CCI, the cortex undergoes neuronal structural degradation in both hemispheres out to 28 days post-injury, which may be indicative of compromised compensatory plasticity. This is likely to be an important consideration in designing therapeutic strategies aimed at enhancing plasticity following TBI.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Adkins D.L. Campos P. Quach D. Borromeo M. Schallert K. Jones T.A. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp. Neurol. 2006;200:356–370. - PubMed
-
- Adkins D.L. Voorhies A.C. Jones T.A. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. - PubMed
-
- Ansari M.A. Roberts K.N. Scheff S.W. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma. 2008b;25:513–526. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

