Multiple functions of microsomal triglyceride transfer protein
- PMID: 22353470
- PMCID: PMC3337244
- DOI: 10.1186/1743-7075-9-14
Multiple functions of microsomal triglyceride transfer protein
Abstract
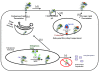
Microsomal triglyceride transfer protein (MTP) was first identified as a major cellular protein capable of transferring neutral lipids between membrane vesicles. Its role as an essential chaperone for the biosynthesis of apolipoprotein B (apoB)-containing triglyceride-rich lipoproteins was established after the realization that abetalipoproteinemia patients carry mutations in the MTTP gene resulting in the loss of its lipid transfer activity. Now it is known that it also plays a role in the biosynthesis of CD1, glycolipid presenting molecules, as well as in the regulation of cholesterol ester biosynthesis. In this review, we will provide a historical perspective about the identification, purification and characterization of MTP, describe methods used to measure its lipid transfer activity, and discuss tissue expression and function. Finally, we will review the role MTP plays in the assembly of apoB-lipoprotein, the regulation of cholesterol ester synthesis, biosynthesis of CD1 proteins and propagation of hepatitis C virus. We will also provide a brief overview about the clinical potentials of MTP inhibition.
Figures

References
-
- Wetterau JR, Zilversmit DB. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J Biol Chem. 1984;259:10863–10866. - PubMed
-
- Wetterau JR, Zilversmit DB. Localization of intracellular triacyglycerol and cholesteryl ester transfer activity in rat tissue. Biochim Biophys Acta. 1986;875:610–617. - PubMed
-
- Wetterau JR, Combs KA, Spinner SN, Joiner BJ. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990;265:9800–9807. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

