Neurotrophin-4 regulates the survival of gustatory neurons earlier in development using a different mechanism than brain-derived neurotrophic factor
- PMID: 22353733
- PMCID: PMC3816093
- DOI: 10.1016/j.ydbio.2012.02.008
Neurotrophin-4 regulates the survival of gustatory neurons earlier in development using a different mechanism than brain-derived neurotrophic factor
Abstract
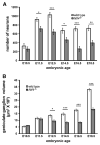
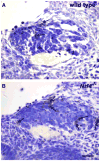
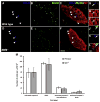
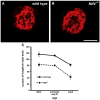
The number of neurons in the geniculate ganglion that are available to innervate taste buds is regulated by neurotrophin-4 (NT-4) and brain-derived neurotrophic factor (BDNF). Our goal for the current study was to examine the timing and mechanism of NT-4-mediated regulation of geniculate neuron number during development. We discovered that NT-4 mutant mice lose 33% of their geniculate neuronal cells between E10.5 and E11.5. By E11.5, geniculate axons have just reached the tongue and do not yet innervate their gustatory targets; thus, NT-4 does not function as a target-derived growth factor. At E11.5, no difference was observed in proliferating cells or the rate at which cells exit the cell cycle between NT-4 mutant and wild type ganglia. Instead, there was an increase in TUNEL-labeling, indicating an increase in cell death in Ntf4(-/-) mice compared with wild types. However, activated caspase-3, which is up-regulated in the absence of BDNF, was not increased. This finding indicates that cell death initiated by NT-4-removal occurs through a different cell death pathway than BDNF-removal. We observed no additional postnatal loss of taste buds or neurons in Ntf4(-/-) mice. Thus, during early embryonic development, NT-4 produced in the ganglion and along the projection pathway inhibits cell death through an activated caspase-3 independent mechanism. Therefore, compared to BDNF, NT-4 plays distinct roles in gustatory development; differences include timing, source of neurotrophin, and mechanism of action.
Published by Elsevier Inc.
Figures




References
-
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Agerman K, Baudet C, Fundin B, Willson C, Ernfors P. Attenuation of a caspase-3 dependent cell death in NT4- and p75-deficient embryonic sensory neurons. Mol Cell Neurosci. 2000;16:258–268. - PubMed
-
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. - PubMed
-
- Al-Hadlaq SM, Bradley RM, MacCallum DK, Mistretta CM. Embryonic genic-ulate ganglion neurons in culture have neurotrophin-specific electrophysiological properties. Neuroscience. 2003;118:145–159. - PubMed
-
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

