Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial
- PMID: 22353950
- PMCID: PMC3345186
- DOI: 10.1097/AOG.0b013e3182475fa4
Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial
Abstract
Objective: To evaluate sexual function in midlife women using selective serotonin reuptake inhibitors for vasomotor symptoms. Selective serotonin reuptake inhibitors effectively treat vasomotor symptoms but adversely affect sexual function in depressed populations. Information on sexual function in nondepressed midlife women using selective serotonin reuptake inhibitors for vasomotor symptoms is lacking; any treatments that might impair function are of concern.
Methods: This was a randomized controlled trial comparing 8 weeks of escitalopram with placebo in women ages 40-62 years with 28 or more bothersome vasomotor symptoms per week. Change in Female Sexual Function Index composite score (ranges from 2 [not sexually active, no desire] to 36) and six sexual domains (desire, arousal, lubrication, orgasm, satisfaction, pain) and the Female Sexual Distress Scale, and a single-question of sexually-related personal distress from the Female Sexual Distress Scale, were compared between groups.
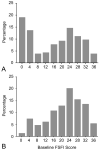
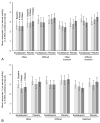
Results: Among all women, median composite baseline Female Sexual Function Index score was 18.1 (interquartile range 2.4-26.5, n=200) and among sexually active women was 22.8 (interquartile range 17.4-27.0, n=75) in the escitalopram group and 23.6 (interquartile range 14.9-31.0, n=70) in the placebo group. Treatment with escitalopram did not affect composite Female Sexual Function Index score at follow-up compared with placebo (P=.18 all women; P=.47 sexually active at baseline). Composite mean Female Sexual Function Index change from baseline to week 8 was 0.1 (95% confidence interval [CI] -1.5 to 1.7) for escitalopram and 2.0 (95% CI 0.2-3.8) for placebo. The Female Sexual Distress Scale results did not differ between groups (P=.73) nor did adverse reports of sexual function. At week 8, among those women sexually active at baseline, there was a small difference between groups in Female Sexual Function Index domain mean score change in lubrication (P=.02) and a marginal nonsignificant difference in orgasm (P=.07).
Conclusion: Escitalopram, when used in the treatment of vasomotor symptoms, did not worsen overall sexual function among nondepressed midlife women.
Trial registration: ClinicalTrials.gov NCT00894543.
Conflict of interest statement
The other authors did not report any potential conflicts of interest.
Figures

Similar articles
-
Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial.Maturitas. 2012 Dec;73(4):361-8. doi: 10.1016/j.maturitas.2012.09.006. Epub 2012 Sep 30. Maturitas. 2012. PMID: 23031421 Free PMC article. Clinical Trial.
-
Sexual function in women on estradiol or venlafaxine for hot flushes: a randomized controlled trial.Obstet Gynecol. 2014 Aug;124(2 Pt 1):233-241. doi: 10.1097/AOG.0000000000000386. Obstet Gynecol. 2014. PMID: 25004335 Free PMC article. Clinical Trial.
-
Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial.JAMA. 2011 Jan 19;305(3):267-74. doi: 10.1001/jama.2010.2016. JAMA. 2011. PMID: 21245182 Free PMC article. Clinical Trial.
-
Managing vasomotor symptoms in women after cancer.Climacteric. 2019 Dec;22(6):544-552. doi: 10.1080/13697137.2019.1600501. Epub 2019 May 13. Climacteric. 2019. PMID: 31081391 Review.
-
[Efficacy and tolerability of escitalopram in anxiety disorders: a review].Encephale. 2008 Sep;34(4):400-8. doi: 10.1016/j.encep.2008.04.004. Epub 2008 Aug 15. Encephale. 2008. PMID: 18922243 Review. French.
Cited by
-
Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial.Maturitas. 2012 Dec;73(4):361-8. doi: 10.1016/j.maturitas.2012.09.006. Epub 2012 Sep 30. Maturitas. 2012. PMID: 23031421 Free PMC article. Clinical Trial.
-
A Clinical Review on Paroxetine and Emerging Therapies for the Treatment of Vasomotor Symptoms.Int J Womens Health. 2022 Mar 10;14:353-361. doi: 10.2147/IJWH.S282396. eCollection 2022. Int J Womens Health. 2022. PMID: 35300283 Free PMC article. Review.
-
Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause.Menopause. 2014 Oct;21(10):1082-90. doi: 10.1097/GME.0000000000000210. Menopause. 2014. PMID: 24552977 Free PMC article. Clinical Trial.
-
Emotional and sexual concerns in women undergoing pelvic surgery and associated treatment for gynecologic cancer.Transl Androl Urol. 2015 Apr;4(2):169-85. doi: 10.3978/j.issn.2223-4683.2015.04.03. Transl Androl Urol. 2015. PMID: 26816823 Free PMC article. Review.
-
Toward a better measure of midlife sexual function: pooled analyses in nearly 1,000 women participating in MsFLASH randomized trials.Menopause. 2022 Jan 31;29(4):397-407. doi: 10.1097/GME.0000000000001940. Menopause. 2022. PMID: 35102098 Free PMC article.
References
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. - PubMed
-
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. - PubMed
-
- Clayton AH, Pradko JF, Croft HA, Montano CB, Leadbetter RA, Bolden-Watson C, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63:357–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- K01 HL108807/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

