Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial
- PMID: 22353950
- PMCID: PMC3345186
- DOI: 10.1097/AOG.0b013e3182475fa4
Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial
Abstract
Objective: To evaluate sexual function in midlife women using selective serotonin reuptake inhibitors for vasomotor symptoms. Selective serotonin reuptake inhibitors effectively treat vasomotor symptoms but adversely affect sexual function in depressed populations. Information on sexual function in nondepressed midlife women using selective serotonin reuptake inhibitors for vasomotor symptoms is lacking; any treatments that might impair function are of concern.
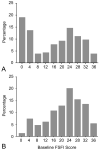
Methods: This was a randomized controlled trial comparing 8 weeks of escitalopram with placebo in women ages 40-62 years with 28 or more bothersome vasomotor symptoms per week. Change in Female Sexual Function Index composite score (ranges from 2 [not sexually active, no desire] to 36) and six sexual domains (desire, arousal, lubrication, orgasm, satisfaction, pain) and the Female Sexual Distress Scale, and a single-question of sexually-related personal distress from the Female Sexual Distress Scale, were compared between groups.
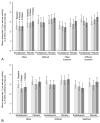
Results: Among all women, median composite baseline Female Sexual Function Index score was 18.1 (interquartile range 2.4-26.5, n=200) and among sexually active women was 22.8 (interquartile range 17.4-27.0, n=75) in the escitalopram group and 23.6 (interquartile range 14.9-31.0, n=70) in the placebo group. Treatment with escitalopram did not affect composite Female Sexual Function Index score at follow-up compared with placebo (P=.18 all women; P=.47 sexually active at baseline). Composite mean Female Sexual Function Index change from baseline to week 8 was 0.1 (95% confidence interval [CI] -1.5 to 1.7) for escitalopram and 2.0 (95% CI 0.2-3.8) for placebo. The Female Sexual Distress Scale results did not differ between groups (P=.73) nor did adverse reports of sexual function. At week 8, among those women sexually active at baseline, there was a small difference between groups in Female Sexual Function Index domain mean score change in lubrication (P=.02) and a marginal nonsignificant difference in orgasm (P=.07).
Conclusion: Escitalopram, when used in the treatment of vasomotor symptoms, did not worsen overall sexual function among nondepressed midlife women.
Trial registration: ClinicalTrials.gov NCT00894543.
Conflict of interest statement
The other authors did not report any potential conflicts of interest.
Figures

References
-
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. - PubMed
-
- Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. - PubMed
-
- Clayton AH, Pradko JF, Croft HA, Montano CB, Leadbetter RA, Bolden-Watson C, et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002;63:357–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- K01 HL108807/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

