Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum
- PMID: 22353963
- PMCID: PMC3327739
- DOI: 10.1097/AOG.0b013e318244ed20
Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum
Abstract
Objective: To estimate the effects of gestational age and other maternal factors on immunologic responses to influenza vaccination.
Methods: Antepartum and postpartum women receiving influenza vaccination as part of routine clinical care were enrolled through four consecutive vaccination seasons (starting October 2006 through January 2010). Immunologic responses to trivalent inactivated influenza vaccine and monovalent H1N1 were assessed as well as factors influencing vaccine responsiveness. Serum samples were obtained at baseline and 4-8 weeks postvaccination.
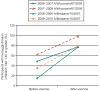
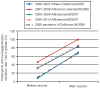
Results: Two hundred thirty-nine participants were included in the current analysis. Seroconversion rates to trivalent inactivated influenza vaccine strains were lowest in the first trimester (54.8%) and immediately postpartum (54.8%) and were highest in the late third trimester (69.6%) and late postpartum (69.4%); these differences were not statistically significant (P=.23). In a multivariable model, higher baseline antibody levels (P<.001) and prior year flu vaccination (P=.03) were both significantly associated with reduced odds of seroconversion. Overall, results were consistent when comparing trivalent inactivated influenza vaccine and monovalent pandemic H1N1 responses. Although there was overall no significant association between gestational age at vaccination (P=.23) or prepregnancy body mass index (P=.16), we observed somewhat lower rates of seroconversion for women vaccinated in the first trimester and for obese women.
Conclusion: Adequate immunologic responses to inactivated influenza vaccines were demonstrated during pregnancy and the postpartum period. No diminution of immunogenicity was observed in the third trimester, a time of increased clinical vulnerability to influenza.
Conflict of interest statement
Figures
Similar articles
-
Immunogenicity of inactivated quadrivalent influenza vaccine in pregnant women, including the level of postvaccination antibodies in umbilical cord blood.Vaccine. 2025 Apr 19;53:127047. doi: 10.1016/j.vaccine.2025.127047. Epub 2025 Apr 8. Vaccine. 2025. PMID: 40203592
-
Safety and immunogenicity of seasonal trivalent inactivated influenza vaccines in pregnant women.Vaccine. 2018 Dec 18;36(52):8054-8061. doi: 10.1016/j.vaccine.2018.10.088. Epub 2018 Nov 8. Vaccine. 2018. PMID: 30416018 Clinical Trial.
-
Similar immunogenicity of the A/H1N1 2009 pandemic influenza strain when used as a monovalent or a trivalent vaccine.Hum Vaccin. 2010 Oct;6(10):823-8. doi: 10.4161/hv.6.10.13600. Epub 2010 Oct 1. Hum Vaccin. 2010. PMID: 20935517 Clinical Trial.
-
Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy.Expert Rev Vaccines. 2012 Aug;11(8):911-21. doi: 10.1586/erv.12.72. Expert Rev Vaccines. 2012. PMID: 23002972 Review.
-
Benefits of influenza vaccination during pregnancy for pregnant women.Am J Obstet Gynecol. 2012 Sep;207(3 Suppl):S17-20. doi: 10.1016/j.ajog.2012.06.070. Epub 2012 Jul 9. Am J Obstet Gynecol. 2012. PMID: 22920053 Free PMC article. Review.
Cited by
-
The Global Influenza Initiative recommendations for the vaccination of pregnant women against seasonal influenza.Influenza Other Respir Viruses. 2015 Aug;9 Suppl 1(Suppl 1):31-7. doi: 10.1111/irv.12320. Influenza Other Respir Viruses. 2015. PMID: 26256293 Free PMC article. Review.
-
Pregnancy and infection.N Engl J Med. 2014 Jun 5;370(23):2211-8. doi: 10.1056/NEJMra1213566. N Engl J Med. 2014. PMID: 24897084 Free PMC article. Review. No abstract available.
-
Vulvovaginal candidiasis in pregnancy.Curr Infect Dis Rep. 2015 Jun;17(6):462. doi: 10.1007/s11908-015-0462-0. Curr Infect Dis Rep. 2015. PMID: 25916994
-
Effects of maternal influenza vaccination on adverse birth outcomes: A systematic review and Bayesian meta-analysis.PLoS One. 2019 Aug 14;14(8):e0220910. doi: 10.1371/journal.pone.0220910. eCollection 2019. PLoS One. 2019. PMID: 31412058 Free PMC article.
-
Obesity Outweighs Protection Conferred by Adjuvanted Influenza Vaccination.mBio. 2016 Aug 2;7(4):e01144-16. doi: 10.1128/mBio.01144-16. mBio. 2016. PMID: 27486196 Free PMC article.
References
-
- Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–8. - PubMed
-
- Louie JK, Acosta M, Jamieson DJ, Honein MA for the California Pandemic (H1N1) Working Goup. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med. 2010;362(1):27–35. - PubMed
-
- Fine AM, Dentinger C, Johnson T, et al. 2009 Pandemic Influenza A (H1N1) in Pregnant Women Requiring Intensive Care - New York City, 2009. MMWR. 2010;59(11):321–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical