Escherichia coli uropathogenesis in vitro: invasion, cellular escape, and secondary infection analyzed in a human bladder cell infection model
- PMID: 22354025
- PMCID: PMC3347433
- DOI: 10.1128/IAI.06075-11
Escherichia coli uropathogenesis in vitro: invasion, cellular escape, and secondary infection analyzed in a human bladder cell infection model
Abstract
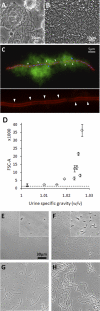
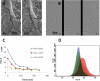
Uropathogenic Escherichia coli (UPEC) strains are capable of invading bladder epithelial cells (BECs) on the bladder luminal surface. Based primarily on studies in mouse models, invasion is proposed to trigger an intracellular uropathogenic cascade involving intracellular bacterial proliferation followed by escape of elongated, filamentous bacteria from colonized BECs. UPEC filaments on the mouse bladder epithelium are able to revert to rod-shaped bacteria, which are believed to invade neighboring cells to initiate new rounds of intracellular colonization. So far, however, these late-stage infection events have not been replicated in vitro. We have established an in vitro model of human bladder cell infection by the use of a flow chamber (FC)-based culture system, which allows investigation of steps subsequent to initial invasion. Short-term bacterial colonization on the FC-BEC layer led to intracellular colonization. Exposing invaded BECs to a flow of urine, i.e., establishing conditions similar to those faced by UPEC reemerging on the bladder luminal surface, led to outgrowth of filamentous bacteria similar to what has been reported to occur in mice. These filaments were capable of reverting to rods that could invade other BECs. Hence, under growth conditions established to resemble those present in vivo, the elements of the proposed uropathogenic cascade were inducible in a human BEC model system. Here, we describe the model and show how these characteristics are reproduced in vitro.
Figures



References
-
- Andersen TE, et al. 2010. A flow chamber assay for quantitative evaluation of bacterial surface colonization used to investigate the influence of temperature and surface hydrophilicity on the biofilm forming capacity of uropathogenic Escherichia coli. J. Microbiol. Methods 81:135–140 - PubMed
-
- Anderson GG, et al. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107 - PubMed
-
- Beloin C, et al. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51:659–674 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

