Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages
- PMID: 22354029
- PMCID: PMC3347434
- DOI: 10.1128/IAI.06120-11
Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages
Abstract
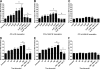
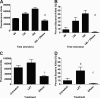
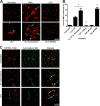
Human and bovine neutrophils release neutrophil extracellular traps (NETs), which are protein-studded DNA matrices capable of extracellular trapping and killing of pathogens. Recently, we reported that bovine neutrophils release NETs in response to the important respiratory pathogen Mannheimia haemolytica and its leukotoxin (LKT). Here, we demonstrate macrophage extracellular trap (MET) formation by bovine monocyte-derived macrophages exposed to M. haemolytica or its LKT. Both native fully active LKT and noncytolytic pro-LKT (produced by an lktC mutant of M. haemolytica) stimulated MET formation. Confocal and scanning electron microscopy revealed a network of DNA fibrils with colocalized histones in extracellular traps released from bovine macrophages. Formation of METs required NADPH oxidase activity, as previously demonstrated for NET formation. METs formed in response to LKT trapped and killed a portion of the M. haemolytica cells. Bovine alveolar macrophages, but not peripheral blood monocytes, also formed METs in response to M. haemolytica cells. MET formation was not restricted to bovine macrophages. We also observed MET formation by the mouse macrophage cell line RAW 264.7 and by human THP-1 cell-derived macrophages, in response to Escherichia coli hemolysin. The latter is a member of the repeats-in-toxin (RTX) toxin family related to the M. haemolytica leukotoxin. This study demonstrates that macrophages, like neutrophils, can form extracellular traps in response to bacterial pathogens and their exotoxins.
Figures

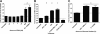



References
-
- Ackermann MR, et al. 1994. Distribution of anti-CD68 (EBM11) immunoreactivity in formalin-fixed, paraffin-embedded bovine tissues. Vet. Pathol. 31:340–348 - PubMed
-
- Aulik N, Hellenbrand K, Kisiela D, Czuprynski C. 2011. Mannheimia haemolytica leukotoxin binds cyclophilin D on bovine neutrophil mitochondria. Microb. Pathog. 50:168–178 - PubMed
-
- Bartneck M, Keul H, Zwadlo-Klarwasser G, Groll J. 2010. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 10:59–63 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

